
Reaction Profile rate-limiting transition state reactants H +Br2 Br-FeBr4 K3.Ioua +FeBr3 intermediate products Br+HBr 10.8 kcal/mol +FeBr3 reaction coordinate
Reaction Profile
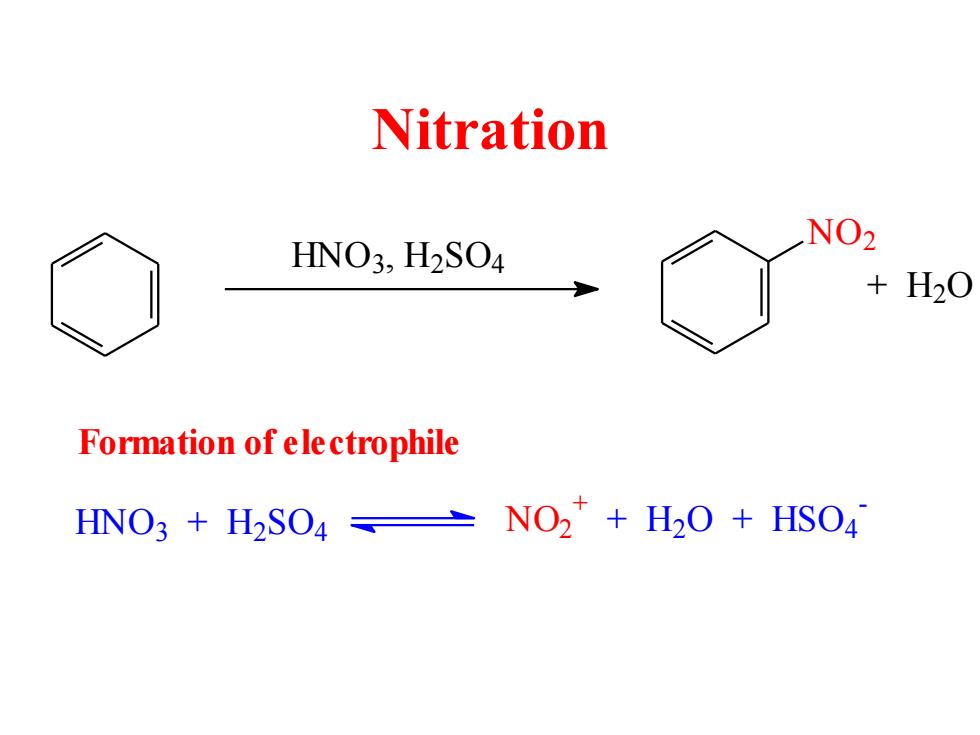
Nitration HNO3,H2SO4 NO2 +H20 Formation of ele ctrophile HNO3 H2SO4 NO2+H2O+HSO4
Nitration HNO3 , H2 SO4 NO2 + H2 O HNO3 + H2 SO4 N O2 + + H2 O + HSO4 - Formation of electrophile
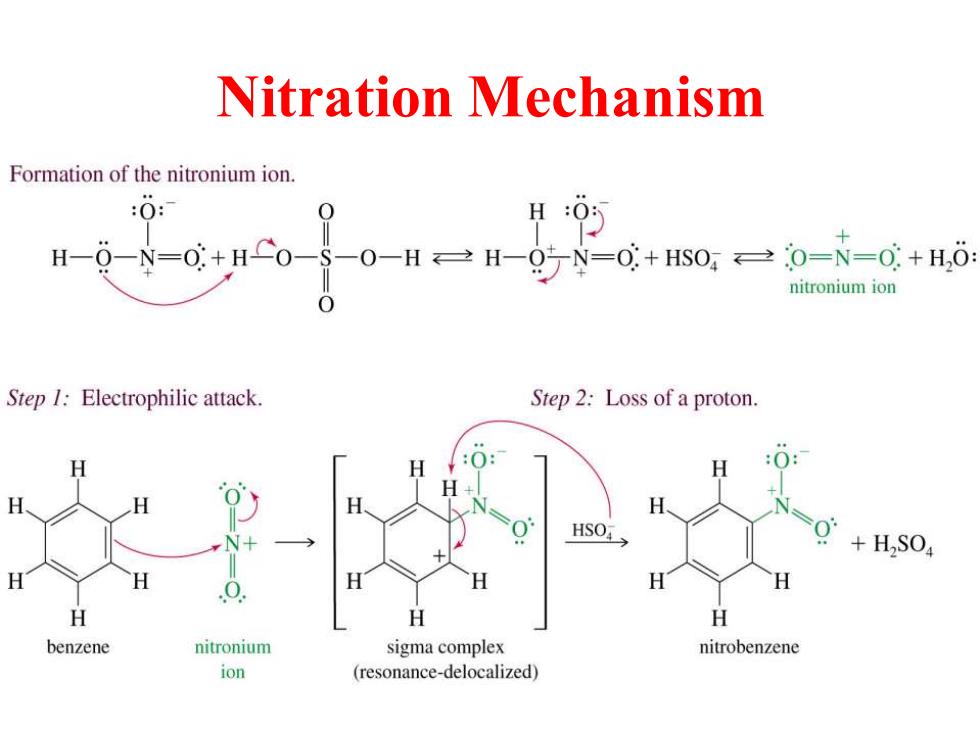
Nitration Mechanism Formation of the nitronium ion. H:0 H--N=+H0-S-0-H=H-95N=+s0=0=N=0+H,6: nitronium ion Step 1:Electrophilic attack. Step 2:Loss of a proton. H H :0: H H H NH HSO> H,SO H H 0 H H H H benzene nitronium sigma complex nitrobenzene ion (resonance-delocalized)
Nitration Mechanism

Nitration of Toluene CH; CH; CH; CH3 HNO3 H2SO NO2 O2N NO2 toluene o-nitrotoluene m-nitrotoluene p-nitrotoluene (60%) (4%) (36%)
Nitration of Toluene

Sulfonation is Reversible fuming sulfuric acid SO3,H2SO4 SO:H SO:H OSO3H
Sulfonation is Reversible H SO3, H2 SO4 SO3H fuming sulfuric acid S O O O H OSO3H SO3H H OSO3H