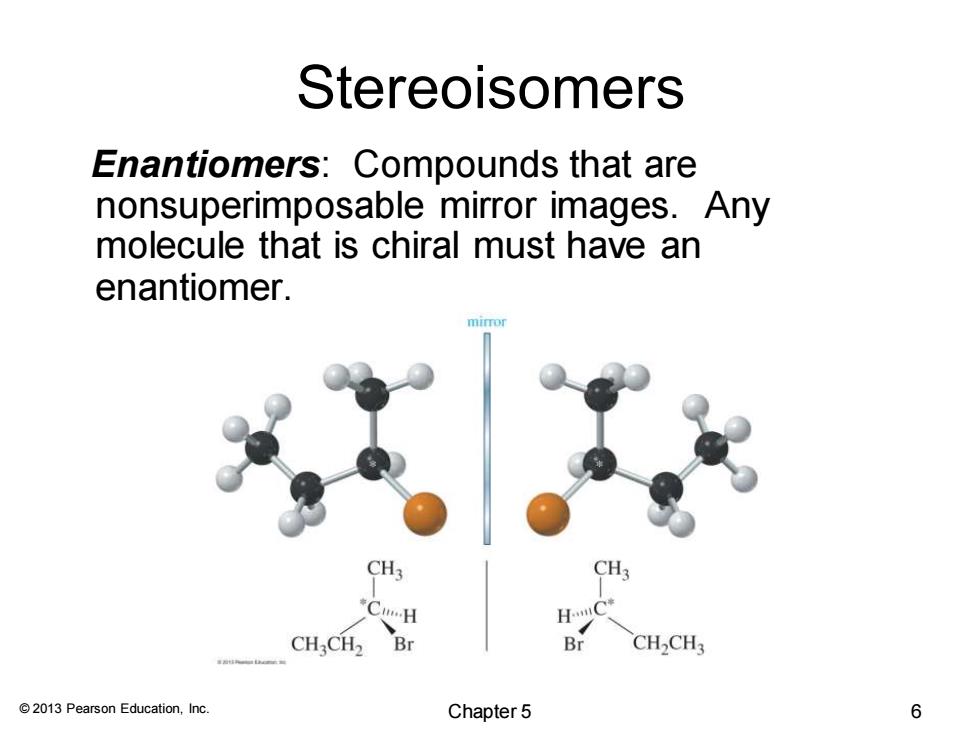
Stereoisomers Enantiomers:Compounds that are nonsuperimposable mirror images.Any molecule that is chiral must have an enantiomer. mimor CH3 CH: CHCH2 Br Br CH>CH3 2013 Pearson Education,Inc. Chapter 5 6
© 2013 Pearson Education, Inc. Stereoisomers Enantiomers: Compounds that are nonsuperimposable mirror images. Any molecule that is chiral must have an enantiomer. Chapter 5 6
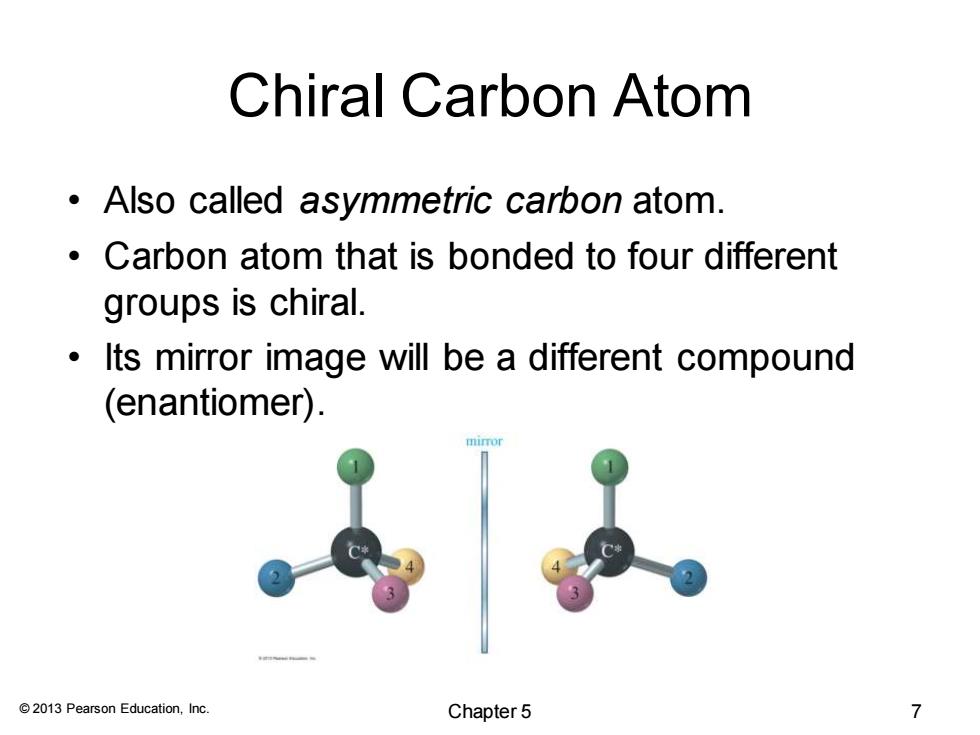
Chiral Carbon Atom Also called asymmetric carbon atom. Carbon atom that is bonded to four different groups is chiral. Its mirror image will be a different compound (enantiomer). 2013 Pearson Education,Inc. Chapter5 7
© 2013 Pearson Education, Inc. Chiral Carbon Atom • Also called asymmetric carbon atom. • Carbon atom that is bonded to four different groups is chiral. • Its mirror image will be a different compound (enantiomer). Chapter 5 7
Stereocenters An asymmetric carbon atom is the most common example of a chirality center. Chirality centers belong to an even broader group called stereocenters.A stereocenter (or stereogenic atom)is any atom at which the interchange of two groups gives a stereoisomer. Asymmetric carbons and the double-bonded carbon atoms in cis-trans isomers are the most common types of stereocenters. 2013 Pearson Education,Inc. Chapter 5 8
© 2013 Pearson Education, Inc. Stereocenters • An asymmetric carbon atom is the most common example of a chirality center. • Chirality centers belong to an even broader group called stereocenters. A stereocenter (or stereogenic atom) is any atom at which the interchange of two groups gives a stereoisomer. • Asymmetric carbons and the double-bonded carbon atoms in cis-trans isomers are the most common types of stereocenters. Chapter 5 8
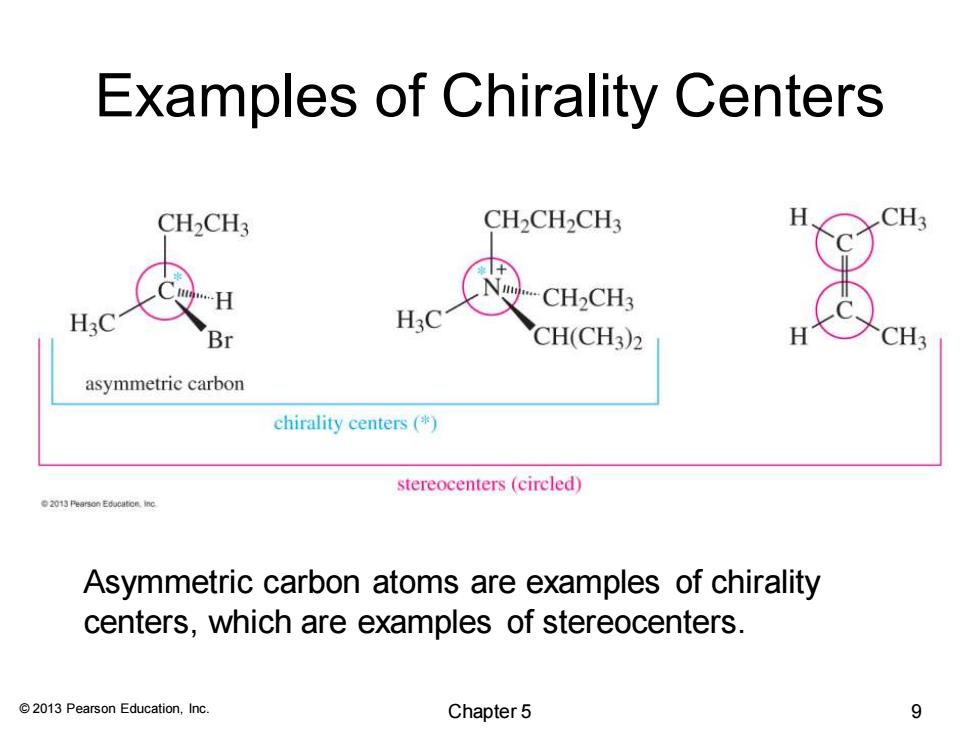
Examples of Chirality Centers CH2CH3 CH2CH2CH3 CH2CH3 H3 H3C Br CH(CH3)2 asymmetric carbon chirality centers (* stereocenters(circled) 2013 Pearson Education inc Asymmetric carbon atoms are examples of chirality centers,which are examples of stereocenters. 2013 Pearson Education,Inc. Chapter 5 9
© 2013 Pearson Education, Inc. Examples of Chirality Centers Asymmetric carbon atoms are examples of chirality centers, which are examples of stereocenters. Chapter 5 9
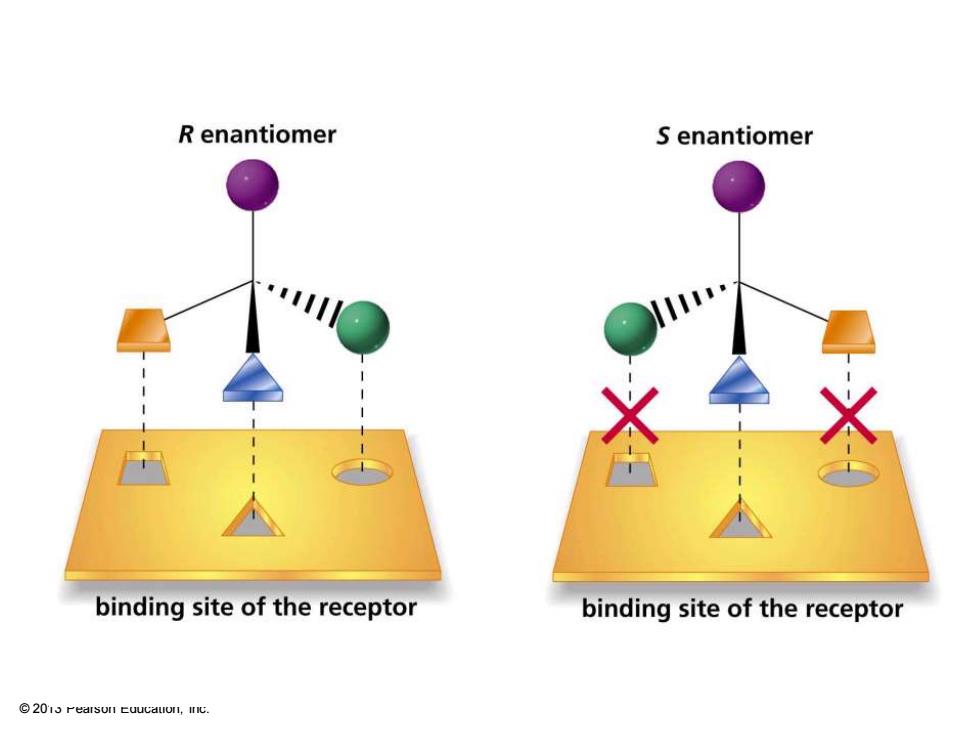
R enantiomer S enantiomer binding site of the receptor binding site of the receptor 2015 Pearson Euucduon,InC
© 2013 Pearson Education, Inc