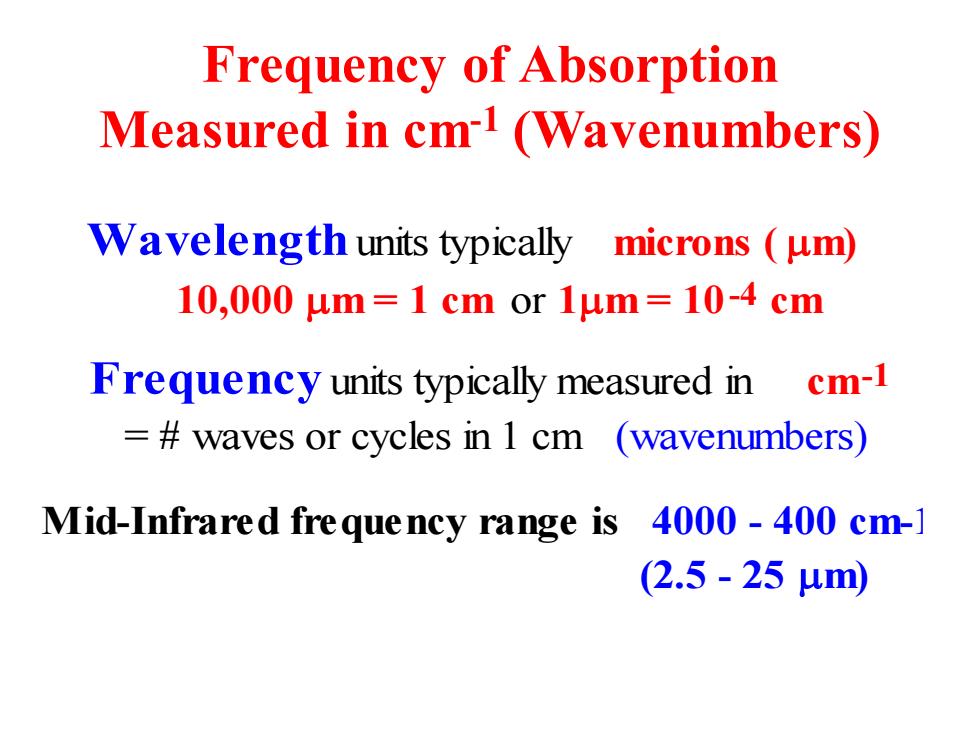
Frequency of Absorption Measured in cm1(Wavenumbers) Wavelength units typically microns (um) 10,000μm=1cmor1μm=10-4cm Frequency units typically measured in cm-1 =waves or cycles in 1 cm (wavenumbers) Mid-Infrared frequency range is 4000-400 cm-] (2.5-25um)
Frequency of Absorption Measured in cm-1 (Wavenumbers) Wavelength units typically microns ( m) 10,000 m = 1 cm or 1m = 10-4 cm Frequency units typically measured in cm-1 = # waves or cycles in 1 cm (wavenumbers) Mid-Infrared frequency range is 4000 - 400 cm-1 (2.5 - 25 m)
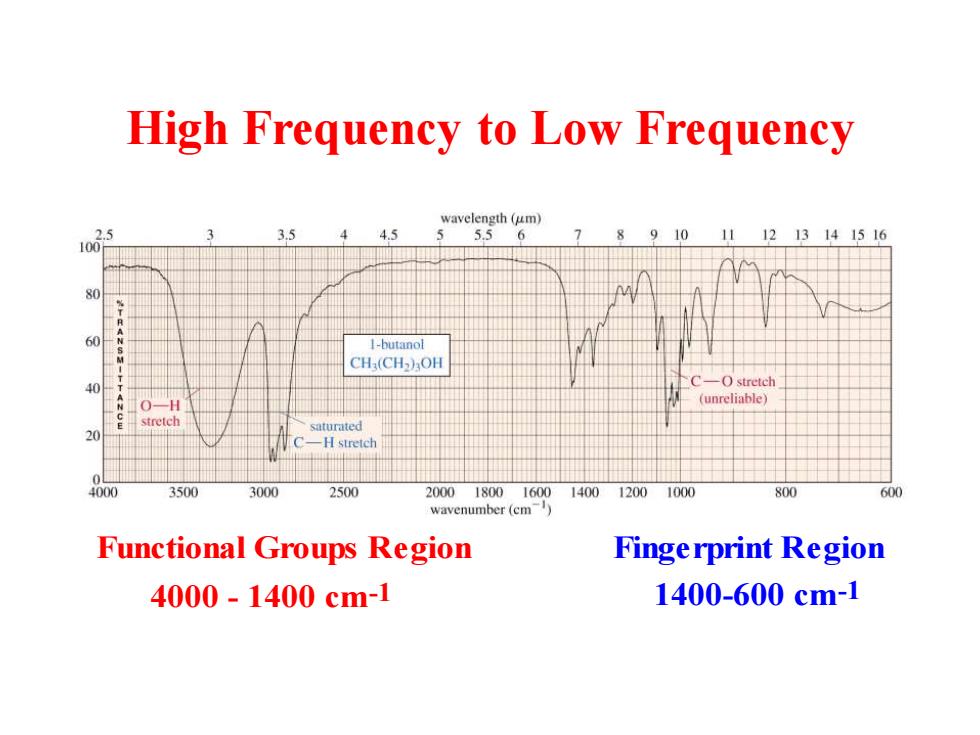
High Frequency to Low Frequency wavelength (um) 5.56 910 1213141516 1-butanol CH(CH3OH -O stretch (unreliable) saturated C-H stretch 4000 3500 3000 2500 200018001600140012001000 800 600 wavenumber(cm) Functional Groups Region Fingerprint Region 4000-1400cm-1 1400-600cm-1
High Frequency to Low Frequency Functional Groups Region 4000 - 1400 cm-1 Fingerprint Region 1400-600 cm-1
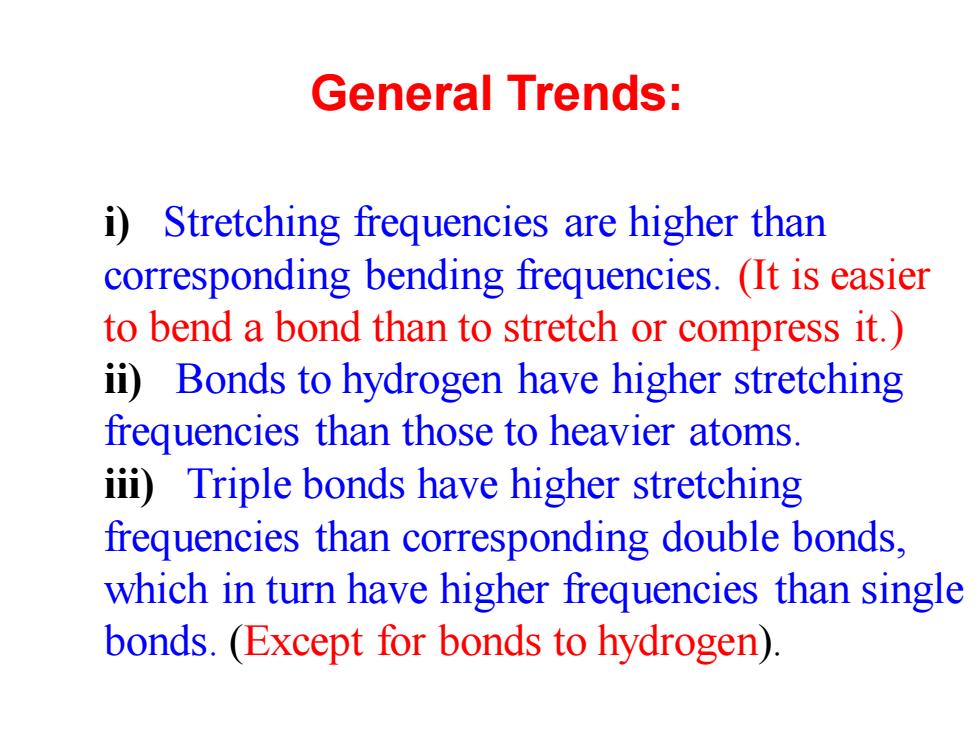
General Trends: i)Stretching frequencies are higher than corresponding bending frequencies.(It is easier to bend a bond than to stretch or compress it.) ii)Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. iii)Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds.(Except for bonds to hydrogen)
General Trends: i) Stretching frequencies are higher than corresponding bending frequencies. (It is easier to bend a bond than to stretch or compress it.) ii) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. iii) Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds. (Except for bonds to hydrogen)
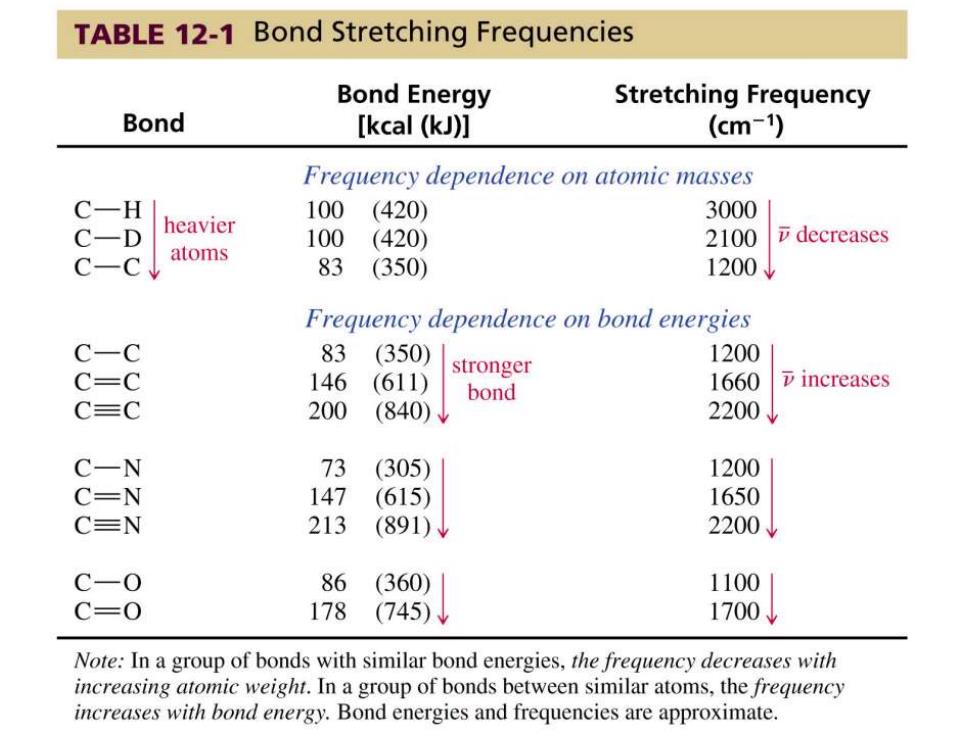
TABLE 12-1 Bond Stretching Frequencies Bond Energy Stretching Frequency Bond [kcal (kJ)] (cm-) Frequency dependence on atomic masses C一H 100(420) 3000 C一D heavier 100(420) 2100 decreases C一C atoms 83 (350) 1200↓ Frequency dependence on bond energies C-C 83 (350) 1200 stronger C=C 146 (611) bond 1660 7 increases CC 200 (840) 2200V C-N 73 (305) 1200 C=N 147 (615) 1650 C=N 213 (891) 2200J C-0 86 (360) 1100 C=0 178 (745)↓ 1700↓ Note:In a group of bonds with similar bond energies,the frequency decreases with increasing atomic weight.In a group of bonds between similar atoms,the frequency increases with bond energy.Bond energies and frequencies are approximate
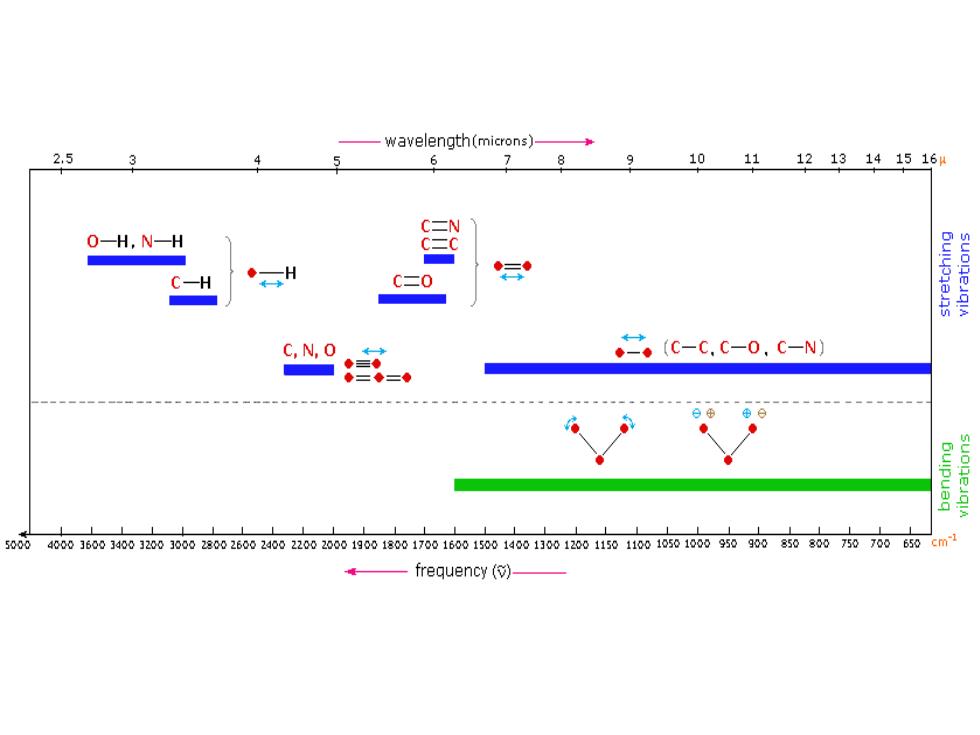
-wavelength(microns)- 2,5 3 8 9 10111213141516 C二N 0-H,N-H C二0 C,N,O ◆-。(C-C.C-0,c-N) = 50084000J60034001200300028002600240020020001900180017001600150014001300120011501001050100095090085080070700650 cm-1 e一frequency()