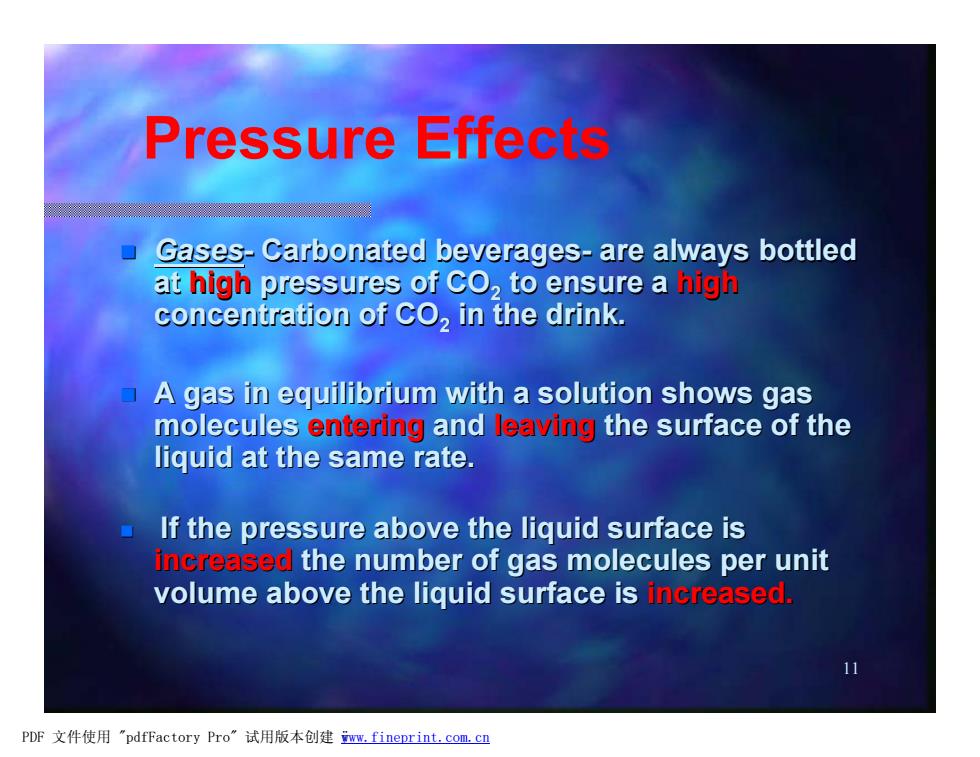
Pressure Effec Gases-Carbonated beverages-are always bottled at high pressures of CO,to ensure a high concentration of CO,in the drink. A gas in equilibrium with a solution shows gas molecules entering and leaving the surface of the liquid at the same rate. If the pressure above the liquid surface is increased the number of gas molecules per unit volume above the liquid surface is increased. 11 PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
11 n Gases- Carbonated beverages- are always bottled at high pressures of CO2 to ensure a high concentration of CO2 in the drink. n A gas in equilibrium with a solution shows gas molecules entering and leaving the surface of the liquid at the same rate. n If the pressure above the liquid surface is increased the number of gas molecules per unit volume above the liquid surface is increased. Pressure Effects PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn
B 12 PDF文件使用"pdfFactory Pro”试用版本创建fw.fineprint.com,cn
12 PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn f

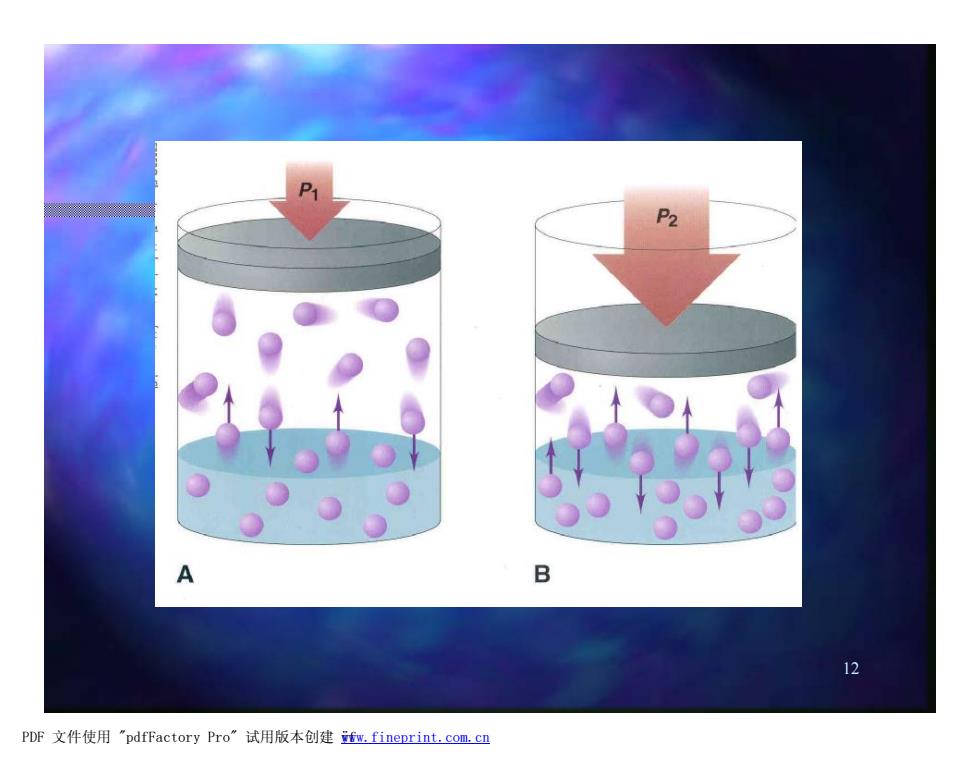
The Effect of Pressure on Gas solubility A.A saturated solution of B.If the pressure is a gas is in equilibrium at increased to P2,the pressure P volume of gas decreases, so the frequency of collisions with the surface increases and more gas is in solution when equilibrium is reestablished. 13 PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
13 n A. A saturated solution of a gas is in equilibrium at pressure P1 n B. If the pressure is increased to P2 , the volume of gas decreases, so the frequency of collisions with the surface increases and more gas is in solution when equilibrium is reestablished. The Effect of Pressure on Gas solubility PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Henry's Law P=kC ---for the solute of dilute solution P is the partial pressure of the gaseous solute above the solution, C represents the concentration of the dissolved gas and k is a constand characteristic of a particular solution The smaller the value of k the more SOLUBLE the gas is. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
14 Henry’s Law n P is the partial pressure of the gaseous solute above the solution, n C represents the concentration of the dissolved gas and n k is a constant, characteristic of a particular solution n The smaller the value of k the more SOLUBLE the gas is. P = kC --- for the solute of dilute solution PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn

Deep Sea Diving He instead of N, ▣k Helium 2700 atm M-1 Nitrogen 1600 atm M-1 Carbon dioxide 29 atm M-1 At 100ft,the air in the lungs is around 4 atm,which causes a large amount of N2 and O2 to dissolve in the blood. If diver ascends too quickly the N,is not released quickly enough and so the diver suffers the bends. He on the other hand is only halr as soluble and so less must be released avoiding this problem. 15 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint..com,c里
15 Deep Sea Diving He instead of N2 n k Helium 2700 atm M-1 Nitrogen 1600 atm M-1 Carbon dioxide 29 atm M-1 n At 100ft, the air in the lungs is around 4 atm, which causes a large amount of N2 and O2 to dissolve in the blood. n If diver ascends too quickly the N2 is not released quickly enough and so the diver suffers the bends. n He on the other hand is only half as soluble and so less must be released avoiding this problem. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿÿwww.fineprint.com.cn