
The Vapour Pressure Of Solutions A solutions vapour pressure is affected by the concentration of solute and the interactions of solute and solvent. salt to melt ice on a footpath-causes the freezing point of the water to decrease thus ice melts if T=0C add a to the water in the radiator of a car to lower the freezing point of the water. The presence of a non-volatile solute lowers the vapour pressure of a solvent. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
6 The Vapour Pressure Of Solutions n A solutions vapour pressure is affected by the concentration of solute and the interactions of solute and solvent. n salt to melt ice on a footpath- causes the freezing point of the water to decrease - n thus ice melts if T = 0oC n add antifreeze to the water in the radiator of a car to lower the freezing point of the water. n The presence of a non-volatile solute lowers the vapour pressure of a solvent. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn
The Effect of Solute on the Vapour Pressure of a Solution A.Equilibrium occurs between a pure liquid and its vapour when the numbers of molecules vaporising and condensing in a given time are equal. Rate of vaporisation Rate of condensation B.With a non-volatile solute present,fewer solvent molecules vaporise in a given time,so fewer molecules need to condense to balance them.Thus equilibrium is reached at a lower vapour pressure. PDF文件使用"pdfFactory Pro”试用版本创建www.fineprint.com.cn
7 The Effect of Solute on the Vapour Pressure of a Solution n A. Equilibrium occurs between a pure liquid and its vapour when the numbers of molecules vaporising and condens condensing in a given time are equal. n Rate of vaporisation = Rate of condensation n B. With a non-volatile solute present, fewer solvent molecules vaporise in a given time, so fewer molecules need to condense condense to balance them. Thus equilibrium is reached at a lower vapour pressure. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
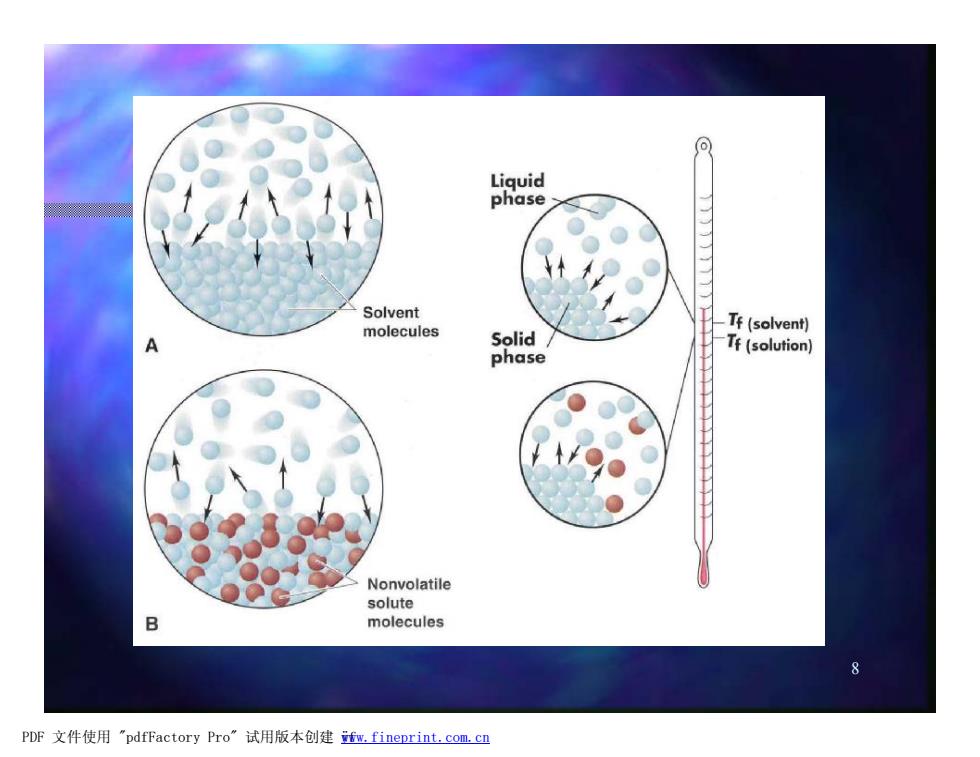
Liquid phase Solvent molecules Solid/ Tf(solvent) Tf(solution) phase Nonvolatile solute B molecules PDF文件使用"pdfFactory Pro”试用版本创建fw.fineprint.com,cn
8 PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn f
The Effect of solute on the vapour pressure A.Equilibrium occurs B.With a nonvolatile between pure liquid solute present,fewer and its vapour when molecules vaporise the number of in a given time,so molecules vaporising fewer molecules and condensing in a molecules need to given time are equal. condense to balance them.Thus the equilibrium is reached at a lower vapour pressure PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,c里
9 The Effect of solute on the vapour pressure n A. Equilibrium occurs between pure liquid and its vapour when the number of molecules vaporising and condensing in a given time are equal. n B. With a nonvolatile solute present, fewer molecules vaporise in a given time, so fewer molecules molecules need to condense to balance them. Thus the equilibrium is reached at a lower vapour pressure PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn

Raoult's Law ▣ Psoln=Xsolvents so is the observed vapour pressure of the solution,xsolvent is the mole fraction of solvent and P"soent is the vapour pressure of the pure solvent. For a solution of half solvent and solute the mole fraction is 0.5 and thus the vapour pressure is decreased by half. The non-volatile solute simply s the solvent. Psoln=Xsolvent solvent --for the solvent of dilute solution 10 PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,c四
10 Raoult’s Law Psoln= csolventP* solvent n Psoln is the observed vapour pressure of the solution, csolvent is the mole fraction of solvent and P*solvent is the vapour pressure of the pure solvent. n For a solution of half solvent and solute the mole fraction is 0.5 and thus the vapour pressure is decreased by half. n The non-volatile solute simply dilutes the solvent. n Psoln= csolventP* solvent --- for the solvent of dilute solution PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn