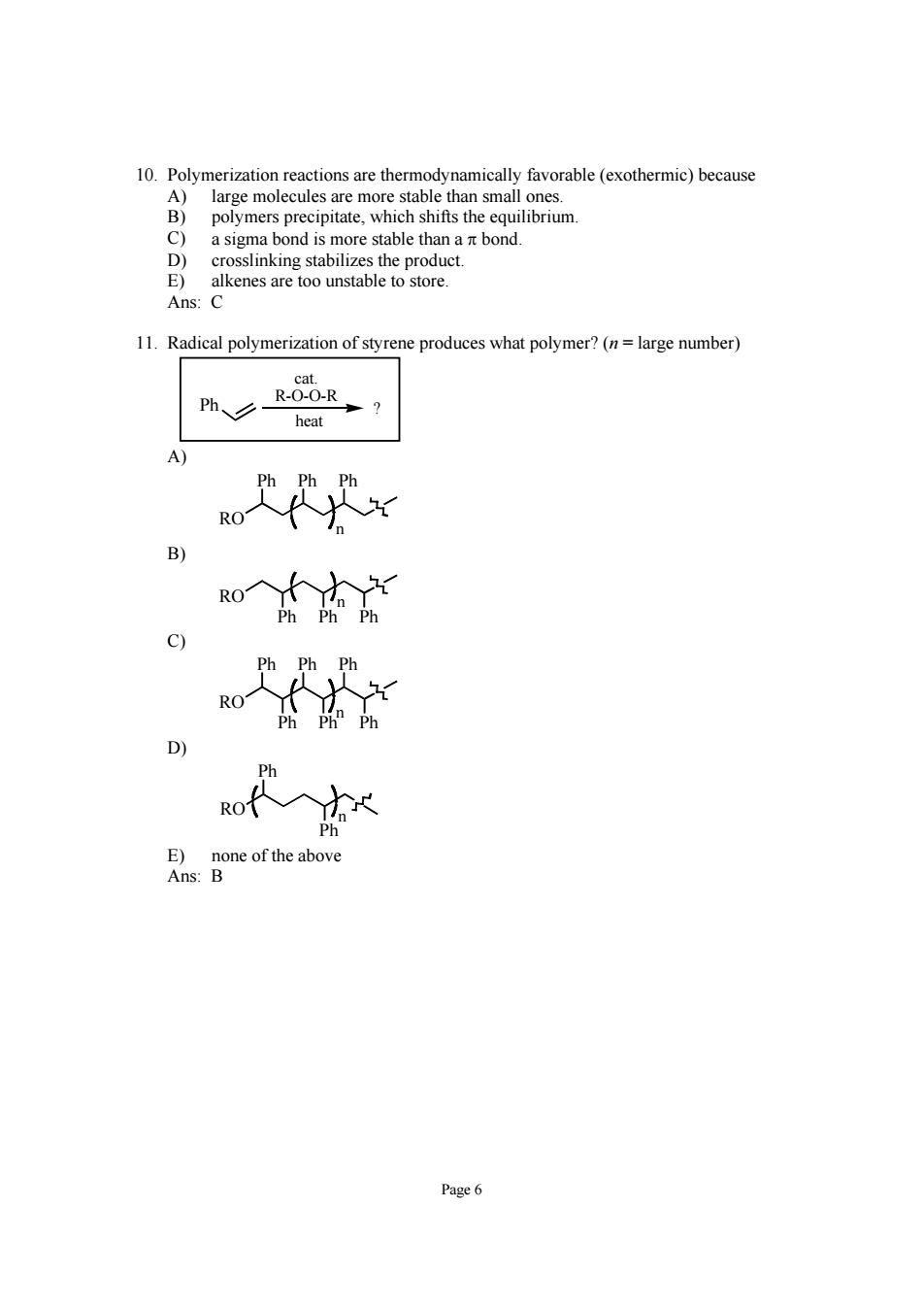
10. large mo stable tha the E alkenes are too unstable to store Ans:C 11.Radical polymerization of styrene produces what polymer?(n=large number) heat o犬代x w入 ofx Page6
Page 6 10. Polymerization reactions are thermodynamically favorable (exothermic) because A) large molecules are more stable than small ones. B) polymers precipitate, which shifts the equilibrium. C) a sigma bond is more stable than a π bond. D) crosslinking stabilizes the product. E) alkenes are too unstable to store. Ans: C 11. Radical polymerization of styrene produces what polymer? (n = large number) Ph ? heat cat. R-O-O-R A) RO Ph Ph Ph n B) RO Ph Ph Ph n C) RO Ph Ph Ph Ph Ph Ph n D) RO Ph Ph n E) none of the above Ans: B
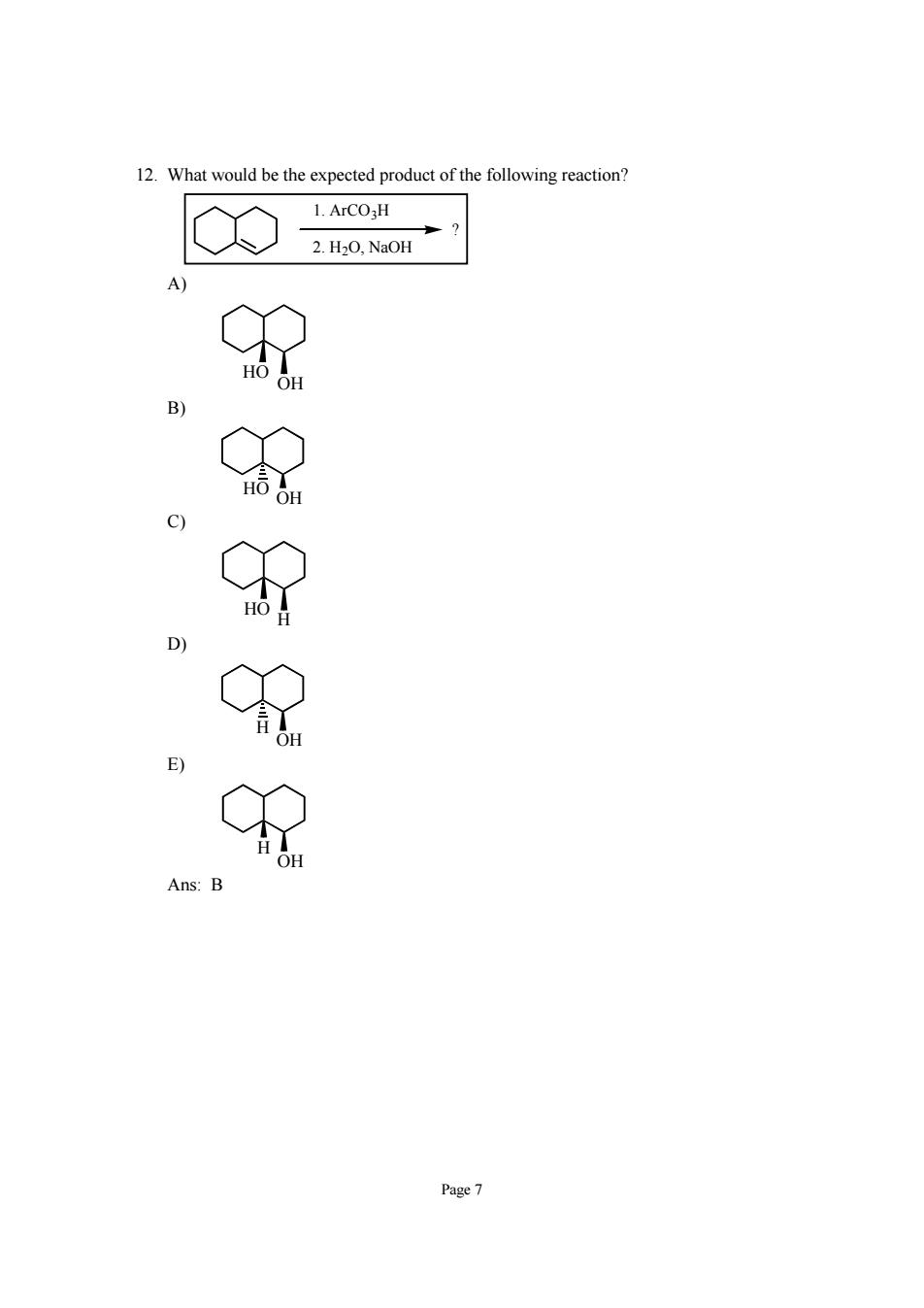
12.What would be the expected product of the following reaction? L ArCO-H 2.H20,Na0H C Page7
Page 7 12. What would be the expected product of the following reaction? ? 1. ArCO3H 2. H2O, NaOH A) OH HO B) OH HO C) H HO D) OH H E) OH H Ans: B
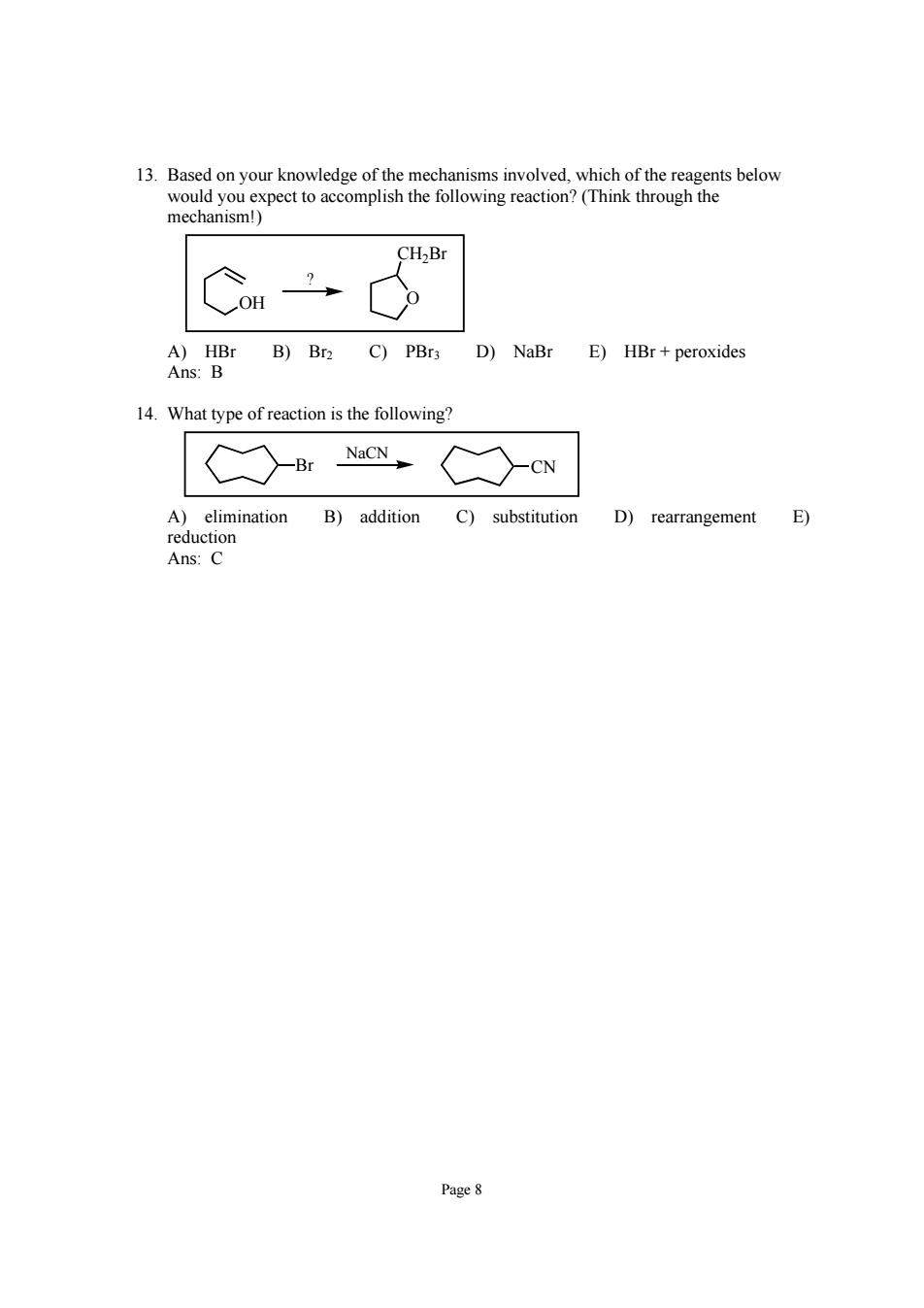
13.Basedon your mechanisms invovd which of the r elow CH2Br B)Br2 C)PBr3 D)NaBr E)HBr+peroxides 14.What type of reaction is the following? h NC-CN D)rearrangement E) Ans:C Page 8
Page 8 13. Based on your knowledge of the mechanisms involved, which of the reagents below would you expect to accomplish the following reaction? (Think through the mechanism!) OH O CH2Br ? A) HBr B) Br2 C) PBr3 D) NaBr E) HBr + peroxides Ans: B 14. What type of reaction is the following? Br CN NaCN A) elimination B) addition C) substitution D) rearrangement E) reduction Ans: C