
Sec 2 Aromaticity and Reactivity 芳香性判别(Hiickel rules) 闭合的共轭体系(环状π电子流) .4n+2 Cyclopentadienyl Anion Planar.. 6πelectrons, therefore aromatic
Sec 2 Aromaticity and Reactivity 芳香性判别(H ückel rules) 闭合的共轭体系 (环状 电子流 ) 4n+2

4πelectrons 6πelectrons 8πelectrons not aromatic aromatic not aromatic ⊕ 6πelectrons Planar... aromatic 6πelectrons

Hiickel's Rule Once the aromatic criteria is met, Huckel's rule applies. If the number of pi electrons is (4N 2)the compound is aromatic (where N is an integer) If the number of pi electrons is (4N) the compound is antiaromatic
Hückel’s Rule Once the aromatic criteria is met, Huckel’s rule applies. If the number of pi electrons is (4N + 2) the compound is aromatic (where N is an integer) If the number of pi electrons is (4N) the compound is antiaromatic
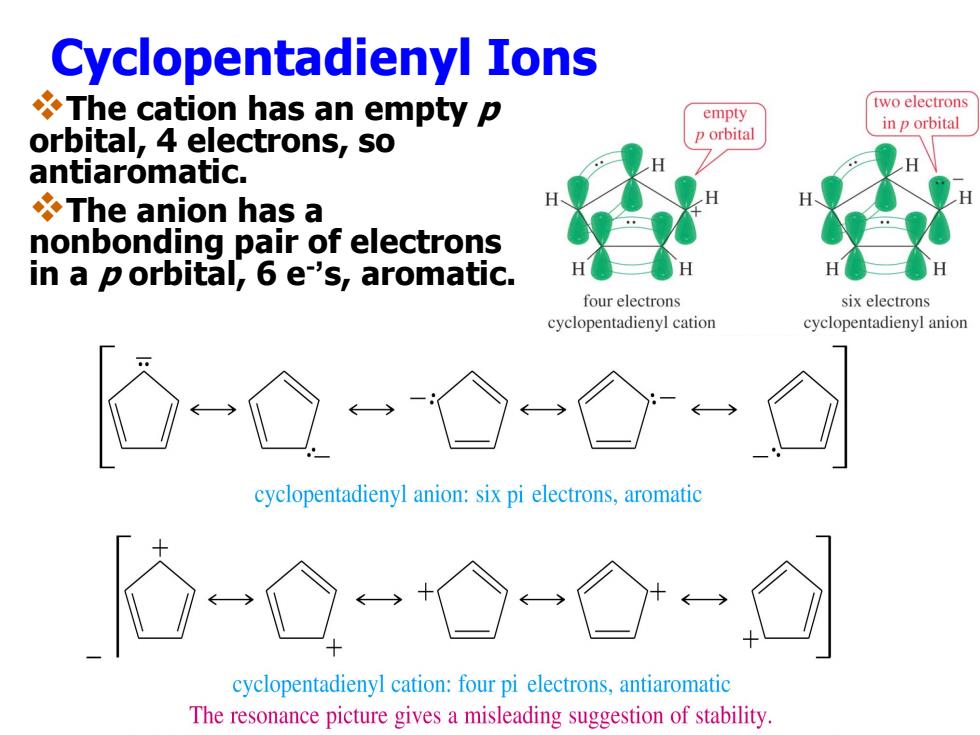
Cyclopentadienyl Ions The cation has an empty p two electrons empty in p orbital orbital,4 electrons,so p orbital antiaromatic. The anion has a nonbonding pair of electrons in a porbital,6 e's,aromatic. four electrons six electrons cyclopentadienyl cation cyclopentadienyl anion cyclopentadienyl anion:six pi electrons,aromatic cyclopentadienyl cation:four pi electrons,antiaromatic The resonance picture gives a misleading suggestion of stability
Cyclopentadienyl Ions The cation has an empty p orbital, 4 electrons, so antiaromatic. The anion has a nonbonding pair of electrons in a p orbital, 6 e - ’s, aromatic

Which of the following is an aromatic compound? Non-aromatic Aromatic There is an sp3 carbon in All carbons are sp3 the ring,delocalization will hybridized and it obeys not be complete. Huckel's rule
Which of the following is an aromatic compound? Non-aromatic Aromatic There is an sp3 carbon in the ring, delocalization will not be complete. All carbons are sp3 hybridized and it obeys Huckel’s rule