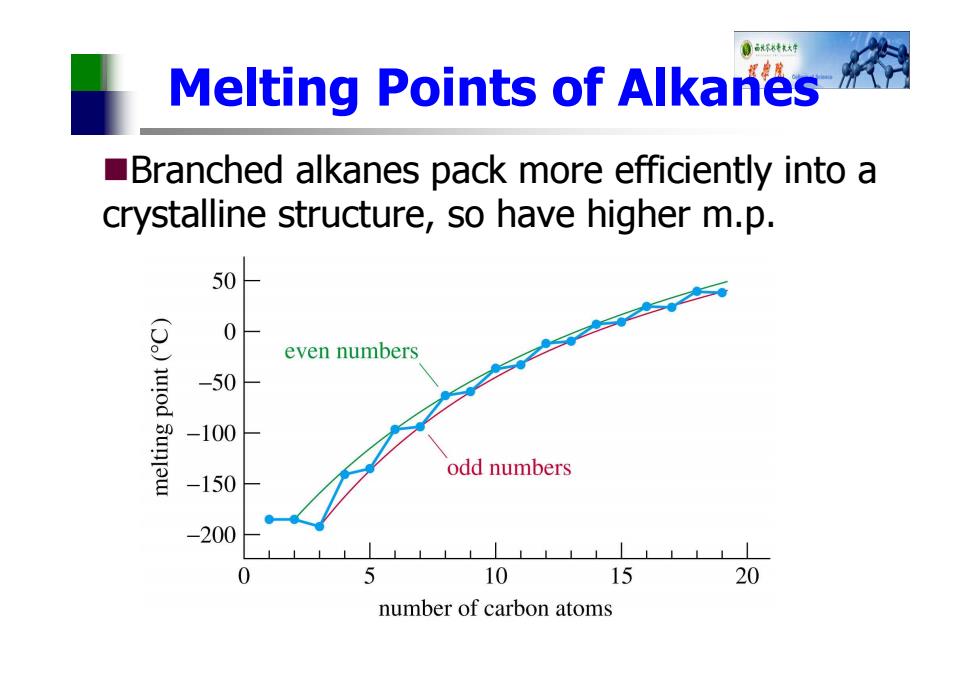
0环行 Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure,so have higher m.p. 50 0 even numbers juod -50 Sunlew -100 odd numbers -150 -200 0 5 10 15 20 number of carbon atoms
Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure, so have higher m.p
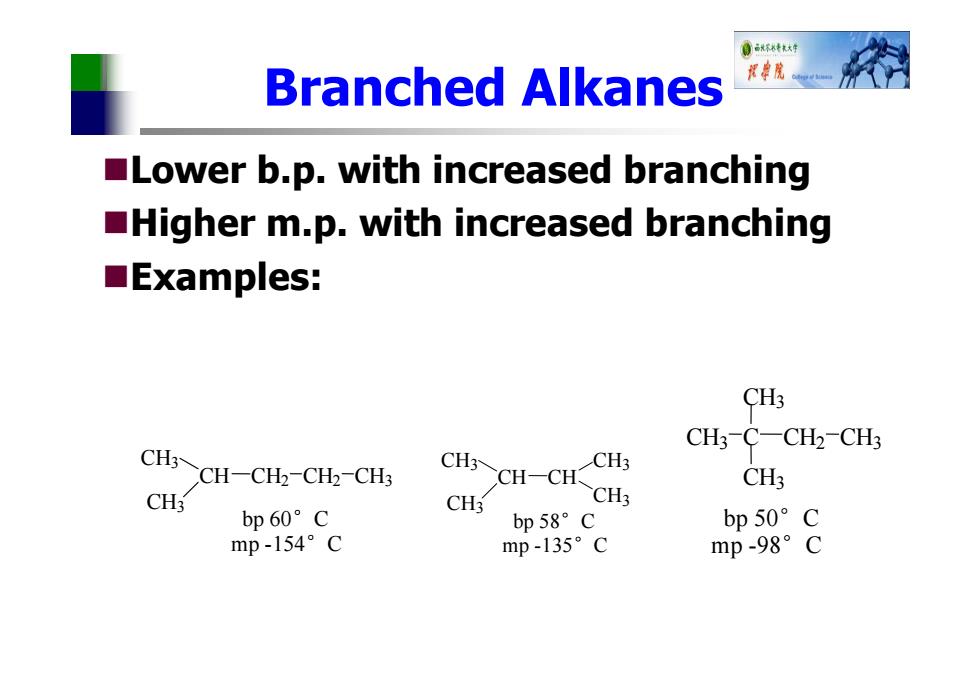
0环+ Branched Akanes 授院。 Lower b.p.with increased branching Higher m.p.with increased branching ■Examples: CH3 CH3-C-CH2-CH3 CH-CH-CHz-CH2-CHs CH CH-CH CHS CH3 CH3 CH3 CH3 bp60°C bp58°C bp50°c mp-154°C mp-135°c mp-98°C
Branched Alkanes Lower b.p. with increased branching Higher m.p. with increased branching Examples: H CH 3 CH CH 3 CH 2 CH 2 CH 3 bp 60°C mp -154°C CH 3 CH CH 3 CH CH 3 CH 3 bp 58°C mp -135°C bp 50 ° C mp -98 ° C CH 3 C C 3 CH 3 CH 2 CH 3

0就不t Major Uses of Alkanes C-C2:gases (natural gas) C3-C4:liquified petroleum (LPG) ■Cs-Cg:gasoline C-C16:diesel,kerosene,jet fuel C17-up:lubricating oils,heating oil Origin:petroleum refining
Major Uses of Alkanes C 1-C 2: gases (natural gas) C 3-C 4: liquified petroleum (LPG) C 5-C 8: gasoline C 9-C16: diesel, kerosene, jet fuel C17-up: lubricating oils, heating oil Origin: petroleum refining
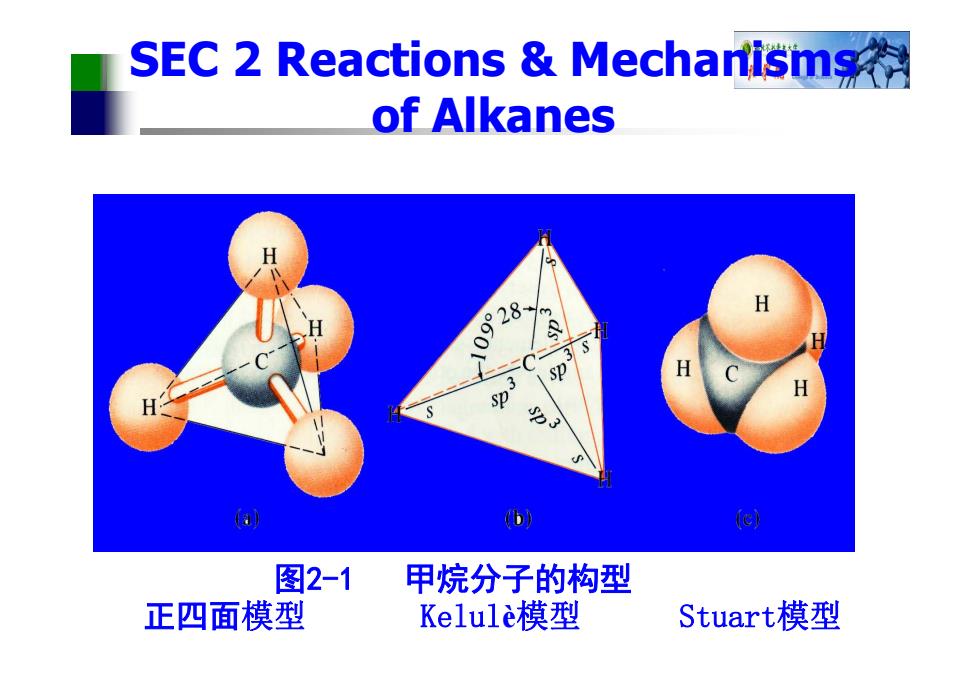
SEC 2 Reactions Mechantisms of Alkanes 09°28 Sp 力3 a (b 图2-1 甲烷分子的构型 正四面模型 Kelule模型 Stuart模型
图2-1 甲烷分子的构型 正四面模型 Kelul è模型 Stuart模型 SEC 2 Reactions & Mechanisms of Alkanes

0 Structure 发华院 Alkane:single bonds,sp3 carbons Cycloalkane:carbons form a ring ■结构特征 CH3-CH3 ■SP杂化碳原子 四面体碳原子 ■σ键相连 ■键合原子可绕σ键轴"自由"旋转 1874 J.H.Van't Hoff Utrecht Univ
Alkane: single bonds, sp3 carbons Cycloalkane: carbons form a ring 结构特征 CH 3 -CH 3 SP3杂化碳原子 四面体碳原子 键相连 键合原子可绕 键轴 “自由 ”旋转 1874 J.H.Van’t Hoff Utrecht Univ Structure