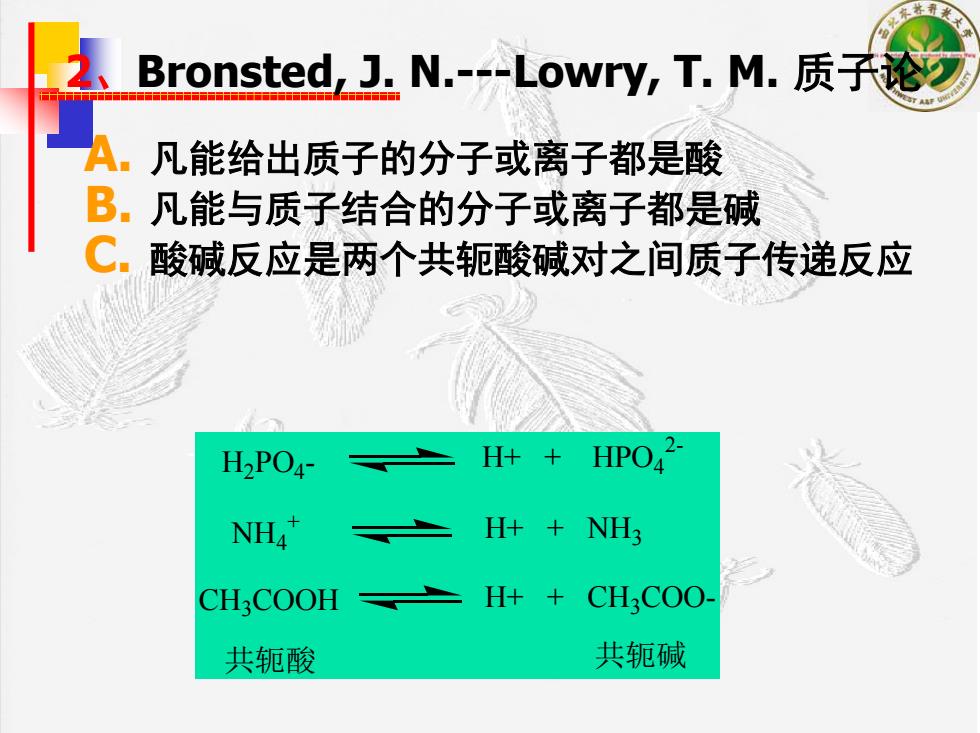
Bronsted,J.N.--Lowry,T.M.质千论 十十 ,凡能给出质子的分子或离子都是酸 B.凡能与质子结合的分子或离子都是碱 C.酸碱反应是两个共轭酸碱对之间质子传递反应 H2PO4- H++ HPO2- NH4 H+NH3 CHCOOH H++ CHCOO 共轭酸 共轭碱
2、Bronsted, J. N.---Lowry, T. M. 质子论 A. 凡能给出质子的分子或离子都是酸 B. 凡能与质子结合的分子或离子都是碱 C. 酸碱反应是两个共轭酸碱对之间质子传递反应 H2PO4- H+ + HPO42- NH4+ H+ + NH3 CH3COOH H+ + CH3COO- 共轭酸 共轭碱

十十于 Bronsted,J.N.--Lowry,T.M.质子论 质子酸碱理论 质子论离开溶剂而从物质能否授受质子给酸碱下定 义,有机化合物中含有大量的C-H,O-H,N-H, S-H,PH键,因此,可以从质子酸碱的强弱来判 断它们的反应情况。 质子酸碱的强弱可以用pKa的大小比较判断 pKa值越大,则酸性越弱 pKa值越小,则酸性越强
2、Bronsted, J. N.---Lowry, T. M. 质子论 质子酸碱理论 ¾ 质子论离开溶剂而从物质能否授受质子给酸碱下定 义,有机化合物中含有大量的C-H,O-H,N-H, S-H,P-H键,因此,可以从质子酸碱的强弱来判 断它们的反应情况。 ¾ 质子酸碱的强弱可以用pKa的大小比较判断 pKa值越大,则酸性越弱 pKa值越小,则酸性越强
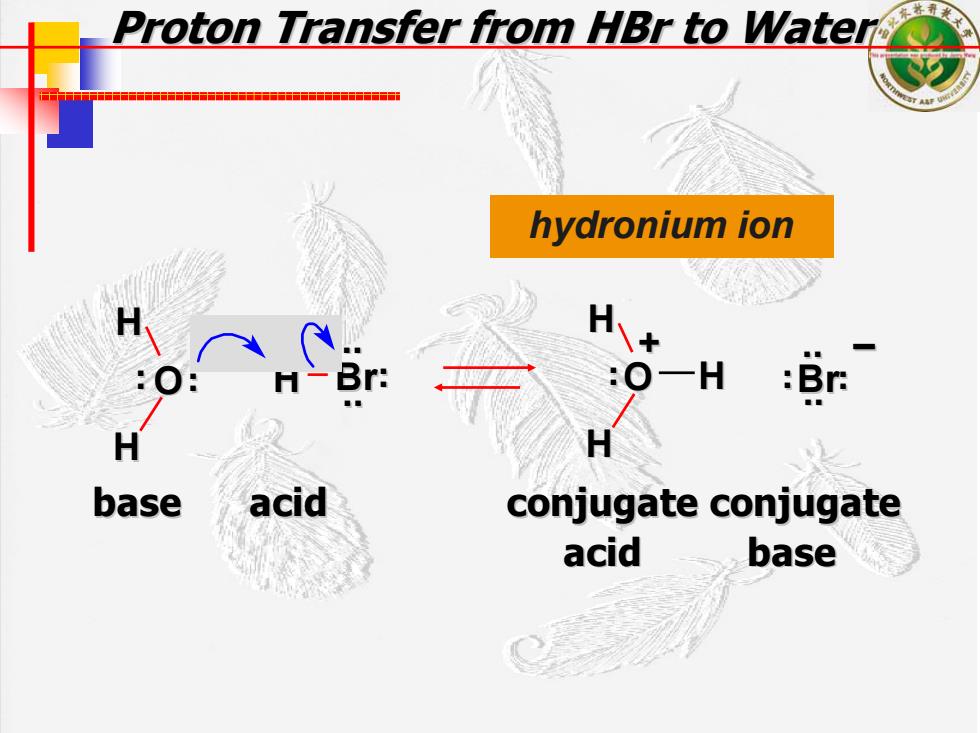
Proton Transfer from HBr to Water 十十 hydronium ion :Br base acid conjugate conjugate acid base
Proton Transfer from Proton Transfer from HBr to Water to Water hydronium ion O H Br H H . . . . H H . .O H Br – . . . . .. .. .. .. . . + base acid conjugate conjugate conjugate conjugate acid base
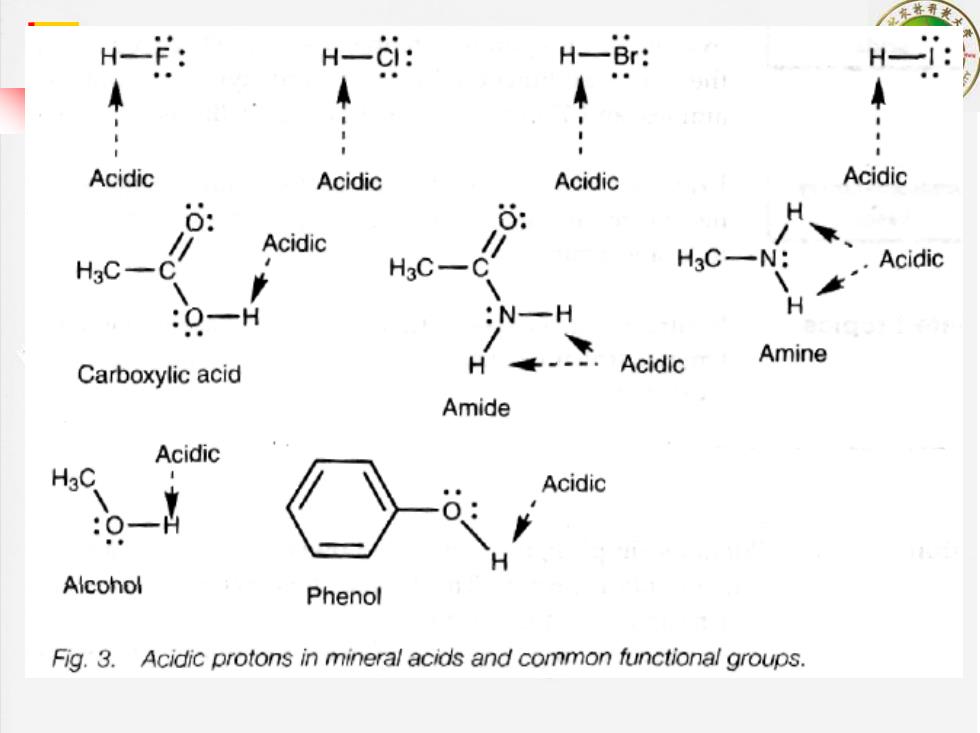
H-: H-CI: HBr: H一 Acidic Acidic Acidic Acidic 6 Acidic HgC H3C-C" H3C-N: Acidic :O-H :N-H Carboxylic acid H Acidic Amine Amide Acidic Acidic Alcohol Phenol Fig.3.Acidic protons in mineral acids and common functional groups
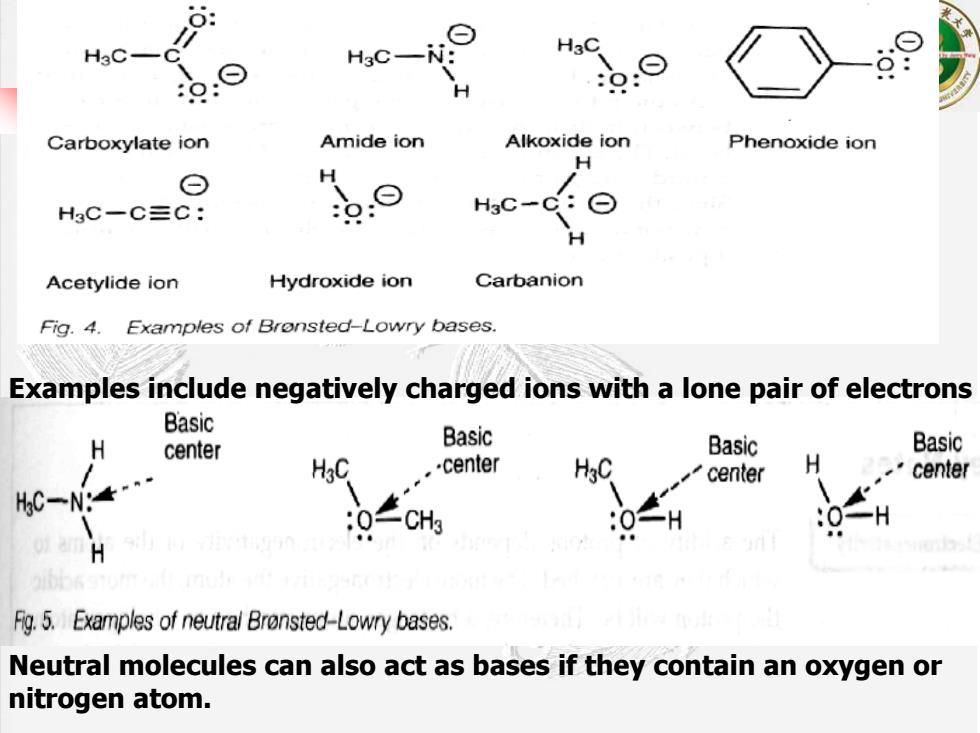
8: H3C- H3C- H Carboxylate ion Amide ion Alkoxide ion Phenoxide ion H ⊙ H HsC-C三C: H3C- Acetylide ion Hydroxide ion Carbanion Fig.4.Examples of Bronsted-Lowry bases. Examples include negatively charged ions with a lone pair of electrons Basic H Basic center Basic Basic HgC -center center H center C一Ng 0H H Fg.5.Examples of neutral Bronsted-Lowry bases. Neutral molecules can also act as bases if they contain an oxygen or nitrogen atom
Neutral molecules can also act as bases if they contain an oxygen or nitrogen atom. Examples include negatively charged ions with a lone pair of electrons