
山东理工大家(形成)常数累积稳定(2)、[ML]M+LEMLβi=K,=[M][L]M+2L←ML[ML2]β,=K,K,-[M][L]?.:1[ML,]M+nL台MILnβ.=K,K,"K.MIL]K表示相邻络合物之间的关系β表示络合物与配体之间的关系16
16 ● ● ● (2)、累积稳定(形成)常数 M+LML [ML] [M][L] 1=K1= M+ 2L ML2 [ML2 ] [M][L]2 2=K1K2= ● ● ● M+nL MLn [MLn ] [M][L]n n =K1K2 ···Kn = K 表示相邻络合物之间的关系 表示络合物与配体之间的关系
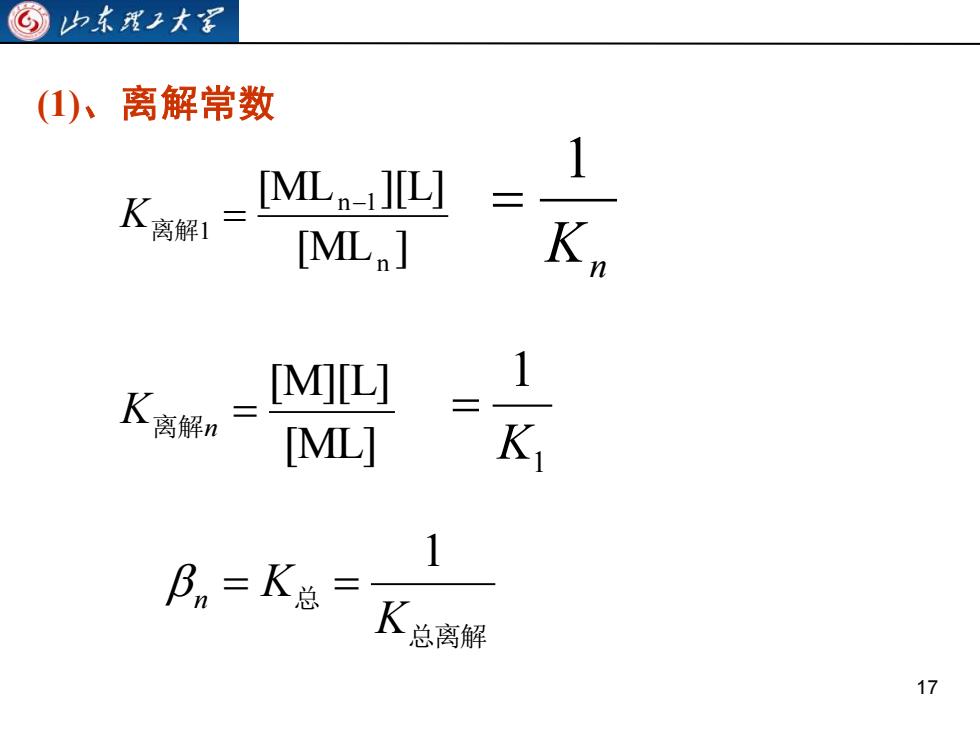
山东理工大家C离解常数(1)、1[ML n-i][L]K离解1K[ML,]n1[M][L]K离解nK,[ML]1β = K总=K总离解17
17 (1)、离解常数 [ML ] [ML ][L] n n 1 1 − K离解 = Kn 1 = [ML] [M][L] = K离解n 1 1 K = 总离解 总 K n K 1 = =

山东理工大家二、溶液中各级配合物的分布:[ML] = β, [MI[L]M+LEMLM+2L←ML[ML2] = β2 [M] [L]2[MLn]= βn[M] [L]"M+nL台MILnCM =[M] +[ML] +[ML2]+..+[ML, ]=[M(1 + β,[L] +β,[L]’ +...+ β,[L]")1[M][M]SM[M] + β,[M][L] +... + β,[M][L]"1+ β[LI+..:+ β.[LI'CM[ML]β,[L]β,[M][L]SMI[M + β[M][L] +...+ β,[M][L]"1+β[L]+...+β,[L]CM18
18 二、溶液中各级配合物的分布: [ML] = 1 [M] [L] [ML2 ] = 2 [M] [L]2 [MLn ]= n [M] [L]n [M] [ML] [ML ] [ML ] [M](1 [L] [L] [L] ) n n 2 c M = + + 2 ++ n = + β1 +β 2 ++ β n 1 n n M 1 n M 1 [L] [L] 1 [M] [M][L] [M][L] [M] [M] c β β + β + + β = + + + = = n 1 n 1 n 1 n 1 M ML 1 [L] [L] [L] [M] [M][L] [M][L] [ML] [M][L] β β β β β β c + + + = + + + = = M+LML M+ 2L ML2 M+nL MLn

山东理工大字Cβ,[L]"[MLn]β,[M[L]"S1+ β[L]+... +β,[L]'CM[M] + β,[M][L] +... + β,[M][L]"[例1]在铜氨溶液中,当氨的平衡浓度为[NH,]=1.00×10-3mol/L时:计算5种形体的分布系数(已知:Cu--NH,配合物lgβ;--β4分别为:4.13,7.61,10.48,12.59)Ccu =[Cu*]+[Cu(NH,)2]+[Cu(NH,),2*]+[Cu(NH,),2*]+[Cu(NH,) 2*]11[Cu]= 1.12%S.89.3211+Zβ,[NH, "Coui=119
19 n 1 n n n n 1 n n n M MLn 1 [L] β [L] [L] [M] [M][L] [M][L] [MLn] [M][L] + + + = + + + = = β β β β β c [例1]在铜氨溶液中,当氨的平衡浓度为 [NH3 ]=1.00×10-3mol/L时: 计算5种形体的分布系数(已知: Cu-NH3配合物lgβ1 -β4分别为:4.13,7.61,10.48,12.59) [Cu ] [Cu(NH ) ] [Cu(NH ) ] [Cu(NH ) ] [Cu(NH ) ] 2 3 4 2 3 3 2 3 2 2 3 2 Cu + + + + + c = + + + + Cu Cu [Cu] c = 1.12% 89.32 1 1 [NH ] 1 1 n i 3 = = + = = n i

山东理工大家C13.49[Cu(NH,]]_β,[NH,]S15.1%Cu(NH,)89.32cc40.70[Cu(NH,)2]_β,[NH,]cu(NH,)245.6%89.32cc30.20[Cu(NH,),]_β,[NH,J3S33.8%Cu(NH)389.32cc3.89β[NH,14[Cu(NH,)4]S4.36%Cu(NH,)489.32cc
20 15.1% 89.32 [Cu(NH3 )] 1 [NH3 ] 13.49 Cu(NH ) 3 = = = = c c 45.6% 89.32 [Cu(NH ) ] [NH ] 40.70 2 3 2 2 3 2 Cu(NH ) 3 = = = = c c 33.8% 89.32 [Cu(NH ) ] [NH ] 30.20 3 3 3 3 3 3 Cu(NH ) 3 = = = = c c 4.36% 89.32 [Cu(NH ) ] [NH ] 3.89 4 3 4 4 3 4 Cu(NH ) 3 = = = = c c