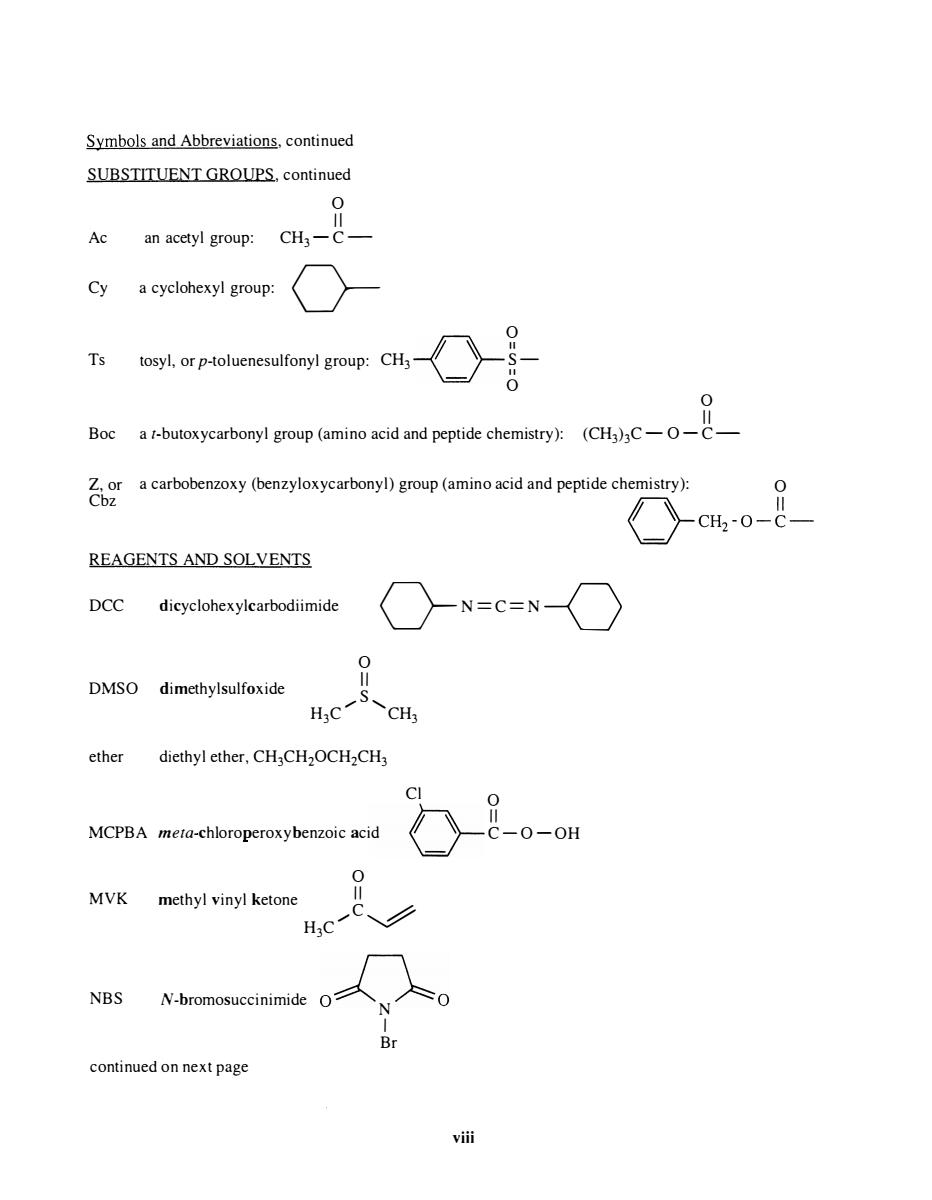
Symbols and Abbreviations.continued SUBSTITUENT GROUPS.continued an acetyl group: CH3-C- 9 a cyclohexyl group: oonroup:cH一《-含力 Ts 0 Boc REAGENTS AND SOLVENTS DCC dicyclohexylcarbodiimide 〉N=C=N〈 DMSO dimethylsulfoxide CH ether diethyl ether,CH3CH2OCH2CH3 MCPBA meta-chloroperoxybenzoic acid -0-0H MVK methyl vinyl ketone NBS N-bromosuccinimide continued on next page vi
Symbols and Abbreviations, continued SUBSTITUENT GROUPS, continued o II Ac an acetyl group: CH3 - C - Cy a cyclohexyl group: 0- o Ts tosyl, or p-toluenesulfonyl group: CH3 � "==1 II S- o o II Boc a t-butoxycarbonyl group (amino acid and peptide chemistry): (CH3)3C -0 - C - Z, or Cbz a carbobenzoxy (benzyloxycarbonyl) group (amino acid and peptide chemistry): 0 REAGENTS AND SOLVENTS DCC dicyclohexylcarbodiimide o-N= C= N-Q DMSO dimethylsulfoxide o II S H3C ....... 'CH3 ether diethyl ether, CH3CH20CH2CH3 Cl 0 MCPBA meta-chloroperoxyhenzo;c ac;d < > g - 0 -OH MVK NBS o methyl vinyl ketone g H3C ....... � N -bromosucci nimide O�O N I Br continued on next page viii < )-CH2-0 - g-

Symbols and Abbreviations,continued REAGENTS AND SOLVENTS,continued PCC pyridinium chlorochromate,CrO3·HC·N CH3H HH CH3 SiaBH disiamylborane H- -S-B-C- C-H CH3 CH3 CH3 CH3 THF tetrahydrofuran SPECTROSCOPY R infrared spectroscopy NMR nuclear magnetic resonance spectroscopy MS mass spectrometry UV ultraviolet spectroscopy parts per million,a unit used in NMR hertz,cycles per second.a unit of frequency MHz megahertz,millions of cycles per second TMS tetramethylsilane,(CH)4Si,the reference compound in NMR s.d.t.q singlet,doublet,triplet.quartet.referring to the number of peaks an NMR absorption gives nm anometers.10meters(usually used as a unit of wavelength m/z mass-to-charge ratio,in mass spectrometry 6 in NMR,chemical shift value,measured in ppm 入 wavelength frequency OTHER a,ax axial (in chair forms of cyclohexane) e,eg equatorial (in chair forms of cyclohexane) HOMO highest occupied molecular orbital LUMO lowest unoccupied molecular orbital NR no reaction o.m.p ortho,meta,para (positions on an aromatic ring) A when written over an arrow:"heat";when written before a letter:"change in" 8,6 partial positive charge,partial negative charge energy from electromagnetic radiation(light) [alp specific rotation at the D line of sodium(589 nm)
Symbols and Abbreviations, continued REAGENTS AND SOLVENTS, continued PCC pyridinium chlorochromate, Cr03· HCI • N� ') CH3 H H H CH3 I I I I I Sia2BH disiamylborane H - C-C-B-C-C-H I I I I CH3 CH3 CH3 CH3 THF tetrahydrofuran o SPECTROSCOPY IR NMR MS UV ppm Hz MHz TMS s, d, t, q nm mJz 8 A v OTHER a, ax e, eq HOMO LUMO NR 0, m,p infrared spectroscopy nuclear magnetic resonance spectroscopy mass spectrometry ultraviolet spectroscopy parts per million, a unit used in NMR hertz, cycles per second, a unit of frequency megahertz, millions of cycles per second tetramethylsilane, (CH3)4Si, the reference compound in NMR singlet, doublet, triplet, quartet, referring to the number of peaks an NMR absorption gives nanometers, 10-9 meters (usually used as a unit of wavelength) mass-to-charge ratio, in mass spectrometry in NMR, chemical shift value, measured in ppm wavelength frequency axial (in chair forms of cyclohexane) equatorial (in chair forms of cyclohexane) highest occupied molecular orbital lowest unoccupied molecular orbital no reaction artha, meta, para (positions on an aromatic ring) when written over an arrow: "heat"; when written before a letter: "change in" partial positive charge, partial negative charge energy from electromagnetic radiation (light) specific rotation at the D line of sodium (589 nm) ix
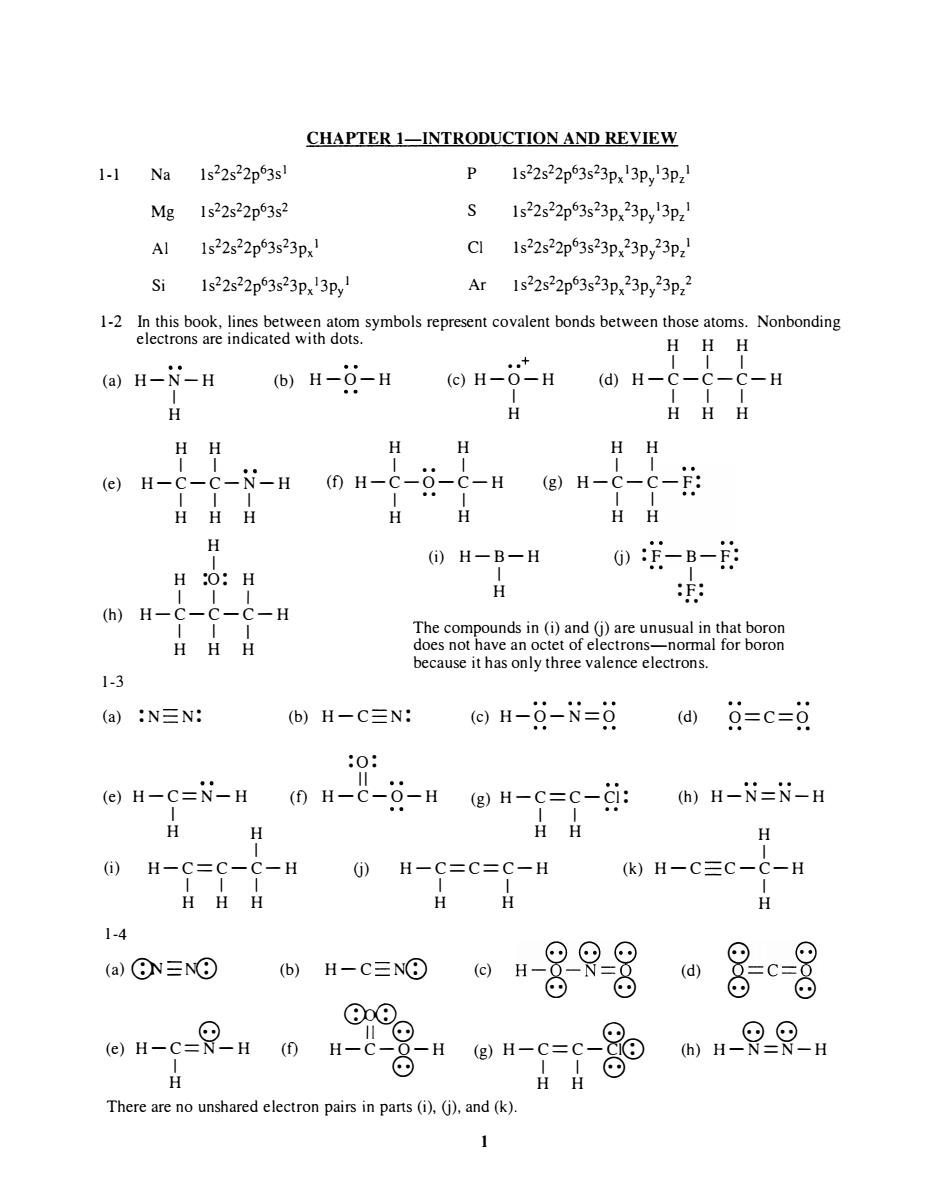
CHAPTER 1-INTRODUCTION AND REVIEW 1-1Na1s22s22p3s P 1s22s22p63s23px3p3pz Mg 1s22s22p63s2 Al 1s22s22p63s23px1 Cl Is22s22p63s23p,23p,23p2 Si 1s22s22p3s23p3p Ar1s22s22p3s23p,23p,23p2 HHH (a)H-N-H (b)H-6-H ⊙H-0-H@H- -H HH HH eH-C---HmH-C--C-HH-C-- HHH H HH H (i)H-B-H 0-B-的 H :O:H H (h)H-C-C-C-H The c HHH vreroorom because it has only three valence electrons. 1-3 (a):N三N: b)H-C三N @)H-9-N=9@)9=c=g eH-C=N-H0H-C-Q-HgH-c=c-年:)H-i=N-H H H 1-4 (a)⑧N三N⊙ 仙-0日8是8 ⊙⊙ on-e-Rn 0 n-8u n-e-g H There are no unshared electron pairs in pars(i).(j).and(k)
1-1 Na Mg AI Si CHAPTER I-INTRODUCTION AND REVIEW 1 s22s22p 6 3s 1 1 s 22s 22p 6 3s2 Is 22s 22p 6 3s 23px 1 1 s22s 22p 6 3s 2 3px 13p y I P S CI Ar 1 s22s 22p 6 3s 2 3px 13p y 13pz 1 1 s22s 22p 6 3s 2 3px 23p y 13pz 1 1 s22s 22p 6 3s 2 3px 23p y 23pz 1 1 s 2 2s22p 6 3s 2 3px 23p y 23pz 2 1-2 In this book, lines between atom symbols represent covalent bonds between those atoms. Nonbonding electrons are indicated with dots. H H H •• + I I I (a) H -N-H I (b) H - O-H (c) H-O-H (d) H-C- C-C-H H H H I I (e) H-C-C- N -H I I I H H H H I H :0: H I I I (h) H-C-C-C-H 1-3 I I I H H H H H I • • I (f) H -C-O-C-H I • • I H H I I I I H H H H H H I I (g) H-C - C - F: I I H H (i) H -B - H I (j) : F-B-F: - - I H : F: .. The compounds in (i) and (j) are unusual in that boron does not have an octet of electrons-normal for boron because it has only three valence electrons. (a) :N N: (b) H - C N: (c) H-O - N=O (d) O==C=O (e) H -C=N- H I H H :0 : II (f) H-C-O-H (g) H -C == C - CI : I I •• H H (h) H -N=N-H H I (i) I H-C==C-C-H (j) H - C==C==C-H (k) H-C C - C-H 1-4 I I I H H H (a) G}J -NQ) 8 (e) H - C=N - H I H (b) (f) H-C NeD C)J(D I I H H (c) 88(.:) H - O-N O 0) 0) • II ·8 H-C-O-H 8 8 (g) H - C == C - CleD I I 8 H H There are no unshared electron pairs in parts (i), (j), and (k). 1 (d) I H 0) 0) o == c=o 0) 0) 88 (h) H-N=N-H
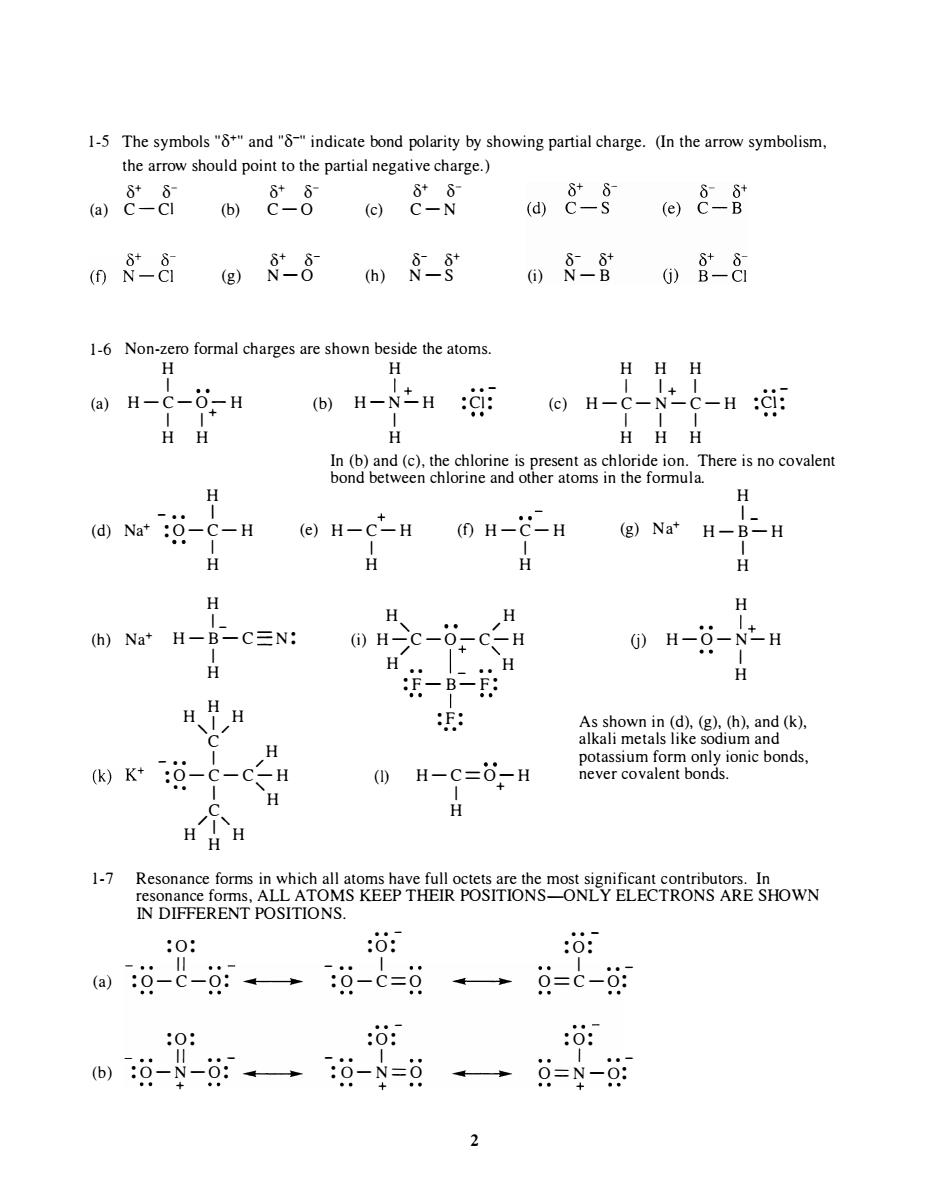
1-5 The symbols"and"indicate bond polarity by showing partial charge.(In the arrow symbolism the arrow should point to the partial negative charge.) 088088 08- 间&§@8-8 0-8g8-8m§-8 0§-含088 1-6 Non-zero formal charges are shown beside the atoms H-N之H: HHH In (b)and (c)the chlorine as chloride ion.There is no covalen bond betwe choeomim he omula H C-H (e)H-C*-H (1)H-C-H (g)Na'H-B-H H H H h)Na*H-B-C三N: H-C-6CH H-O-N-H H H、HH (k)K*-c-c ()H-C=0-H HH H Reeo6N5代6来"零TRONS ARE SHOWN 1-7 Resonance forms in which all aton IN DIFFERENT POSITIONS. :0: :o: :o: a识-c-g:→过-0=9·→总=C-09 0: :o: 过--0。过-8=日一总=9-每 2
1-5 The symbols "8+" and "8-" indicate bond polarity by showing partial charge. (In the arrow symbolism, the arrow should point to the partial negative charge.) � � � � � � (a) C-C1 (b) C - O (c) C-N 1-6 (a) 8+ 8- 8- 8+ (g) N-O (h) N -S Non-zero formal charges are shown beside the atoms. H H 1 1 + .. - H-C-O-H (b) H-N-H : C1: 1 1 + 1 H H H 8- 8+ (i) N-B H 1 H H 1+ 1 (c) H-C-N-C-H 1 1 1 H H H .. - :Cl: In (b) and (c), the chlorine is present as chloride ion. There is no covalent bond between chlorine and other atoms in the formula. H - • • I + H 1- (d) Na+ :O - C - H (e) H-C-H (f) H - C-H 1 (g) Na+ H - B-H (h) Na+ I H H 1 - H-B-C- N: I H H H H ,1/ C H - •• 1 / ·• O- C-C-H • • I ' C H /1' H H H 1 H H H , / H (i) H-C-O-C-H / 1 + H •• _ •• ' H (I) :F-B-F: 1 : F: .. H-C= O- H 1 + H U) I H H 1 H - O-N-H + I H As shown in (d), (g), (h), and (k), alkali metals like sodium and potassium form only ionic bonds, never covalent bonds. 1-7 Resonance forms in which all atoms have full octets are the most significant contributors. In resonance forms, ALL ATOMS KEEP THEIR POSITIONS-ONLY ELECTRONS ARE SHOWN IN DIFFERENT POSITIONS. :0 : " (a) :O- C-O: .. .. :0 : " . . - (b) :O -N-O: .. .. + . . - : 0: - .. 1 :O- C=O . . - :0 : - .. 1 :O- N=O + .. ... 2 .. - : 0: 1 •• - O= C-O: : 0: I •• - O=N - O: +
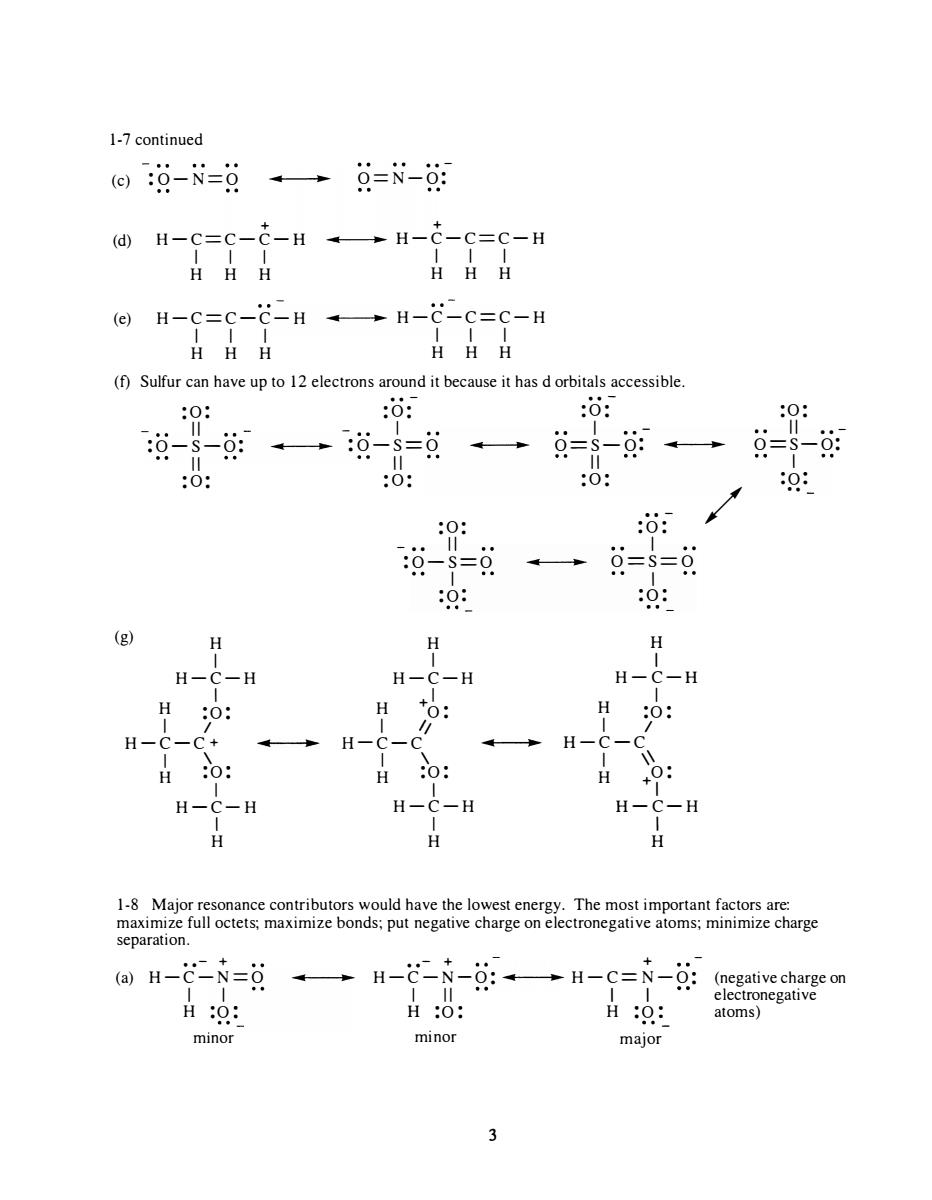
1-7continued 回:0-N=g→9=N-湖 H-c=c-c-H --H-c-c=c-H HHH HHH (f)Sulfur can have upto 12 electrons around it because it has dorbitals accessible. 0: :0: :o: 0: :0: 0 0: 0 9-=9→==g 0: 0: (g) H H H -C-H H -C-H H-C-H H H H 0 H H H 0 H-C-H H-C-H H-C-H H separation. ()H-C-N=8 →H-N-g:→H-C=N-女:((negative H0: H:O: H:0: atoms)gativ minor minor major 3
1-7 continued .. - (c) :O-N=O .. .. O=N-O: + + (d) H-C=C-C-H .. .. H-C-C=C-H I I I I I I H H H H H H (e) H-C=C-C- H .. .. H-C-C=C-H I I I I I I H H H H H H (f) Sulfur can have up to 12 electrons around it because it has d orbitals accessible . (g) : 0: II :O- S- O: II : 0: H I H-C-H I H :0 : I I H-C- C+ I \ H :0 : I H-C-H I H .. .. - : 0: I .. .. :O -S=O .. .. II : 0: H I :0: - •• II :O- S=O I :0: H I H-C-H I + 0: 1/ H- C-C .. I \ H :0 : I H-C-H I H .. . . - :0 : I O==S-O: .. .. II :0 : . . - :0: I .. .. O==S==O I :0 : H I H-C-H I H :0 : I I .. H-C-C I \\ H + 0: I H- C-H I H / :0 : II •• - O=S-O: I :0 : . . 1-8 Major resonance contributors would have the lowest energy. The most important factors are: maximize full octets; maximize bonds; put negative charge on electronegative atoms; minimize charge separation. • • - + . .- + + (a) H-C-N=O .. .. H-C-N-O: .. .. H-C=N- O: (negative charge on I I I II I I electronegative H :0 : H :0 : H :0 : atoms) mInor minor major 3