
实际测量时,都是通过测定由 indicating electrode 和 reference electrode 组成的Cell 的电动势(electromotive force)来完成的。 Reference electrodes 通常由以下几种电极来充当 ,它们的电位在 一定条件下是恒定的,与待测离子浓度无关。 甘汞电极 Ag/AgCl电极 氢电极 pH玻璃电极 Setup of potentiometric methods Frame of reference electrodes in common use (滞后作用,磁滞现象))
实际测量时,都是通过测定由 indicating electrode 和 reference electrode 组成的Cell 的电动势(electromotive force)来完成的。 Reference electrodes 通常由以下几种电极来充当 ,它们的电位在 一定条件下是恒定的,与待测离子浓度无关。 甘汞电极 Ag/AgCl电极 氢电极 pH玻璃电极 Setup of potentiometric methods Frame of reference electrodes in common use (滞后作用,磁滞现象))
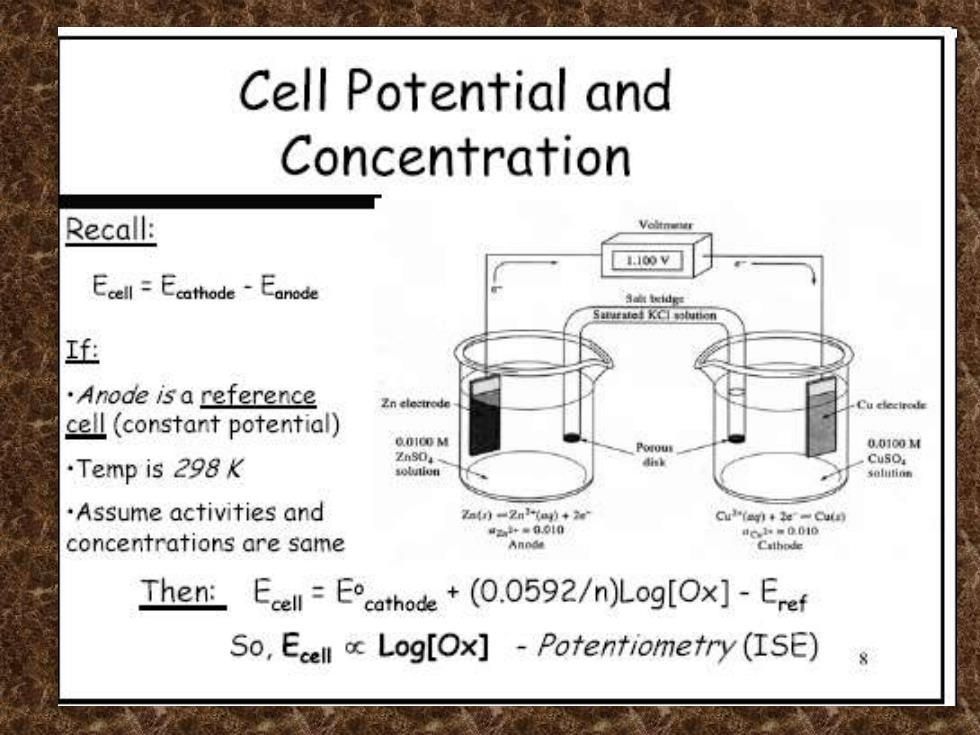
Cell Potential and Concentration Recall: 110oV Ecell =EcathodeEonode Salt teidge f .Anode is a reference Cu clectrode cell(constant potential) 00100 M 0.0100M .Temp is 298K ZnSO CuSO Assume activities and 7atr)-2nor)+2e Cu(ag)2e-Cul “2+=0010 concentrations are same 4.m0010 Cathode Then:Ecell=Ecathode+(0.0592/n)Log[Ox]-Eref So,Ecell o Log[Ox]-Potentiometry(ISE)
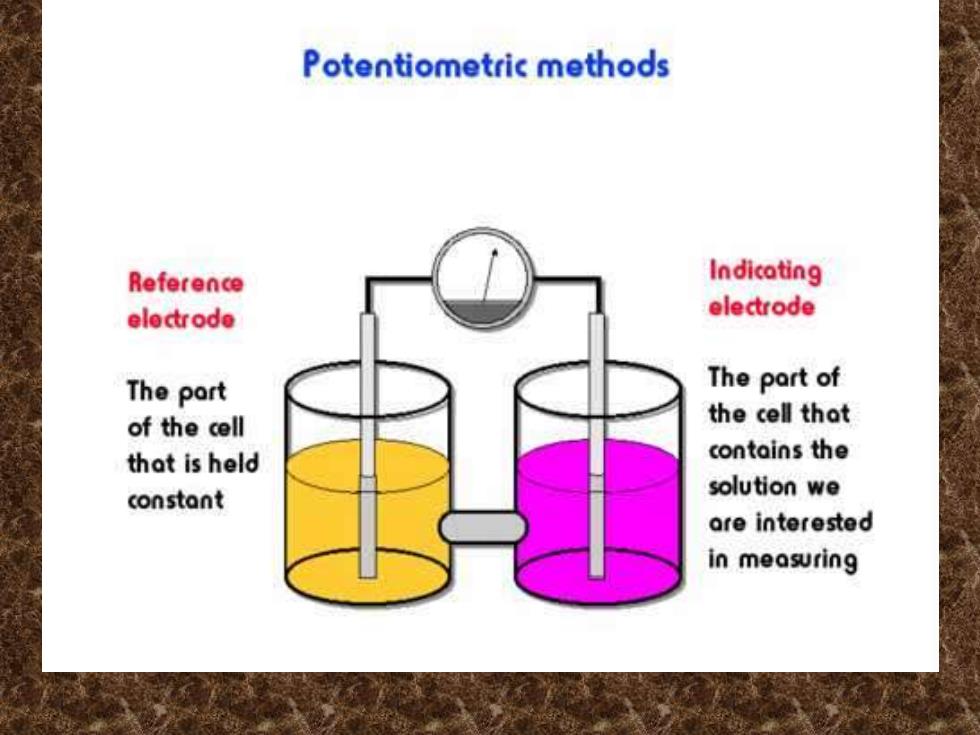
Potentiometric methods Reference Indicating electrode electrode The part The part of of the cell the cell that that is held contains the constant solution we are interested in measuring
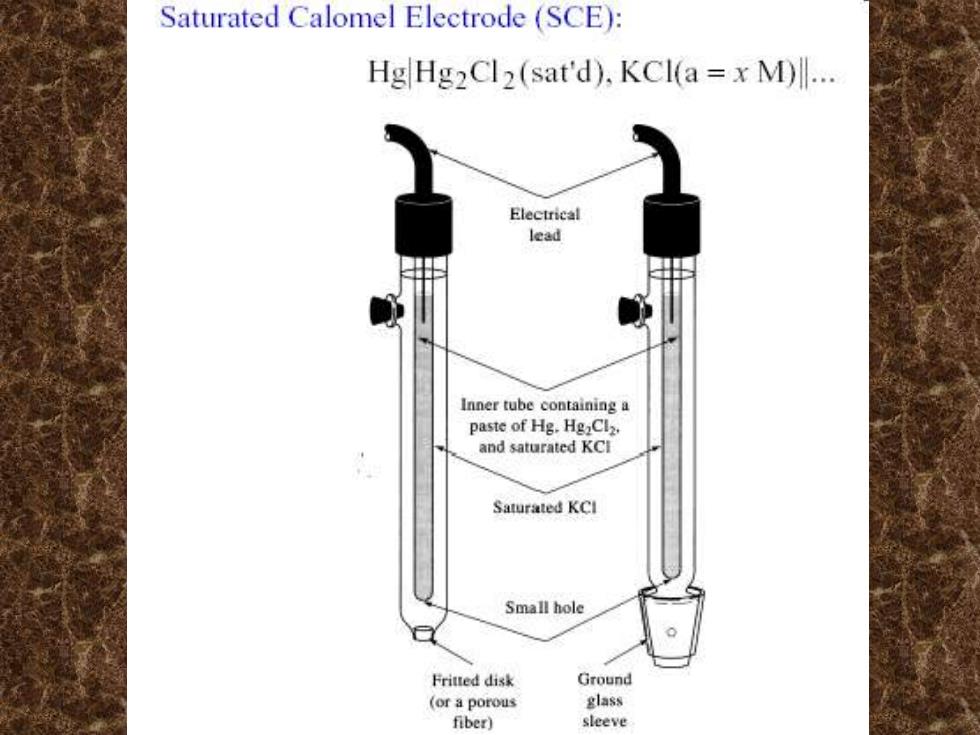
Saturated Calomel Electrode (SCE): HgHg2Cl2(sat'd),KCI(a=x M)l. Electrical lead Inner tube containing a paste of Hg.HgaClz. and saturated KCI Saturated KCl Small hole Fritted disk Ground (or a porous glass fiber) sleeve
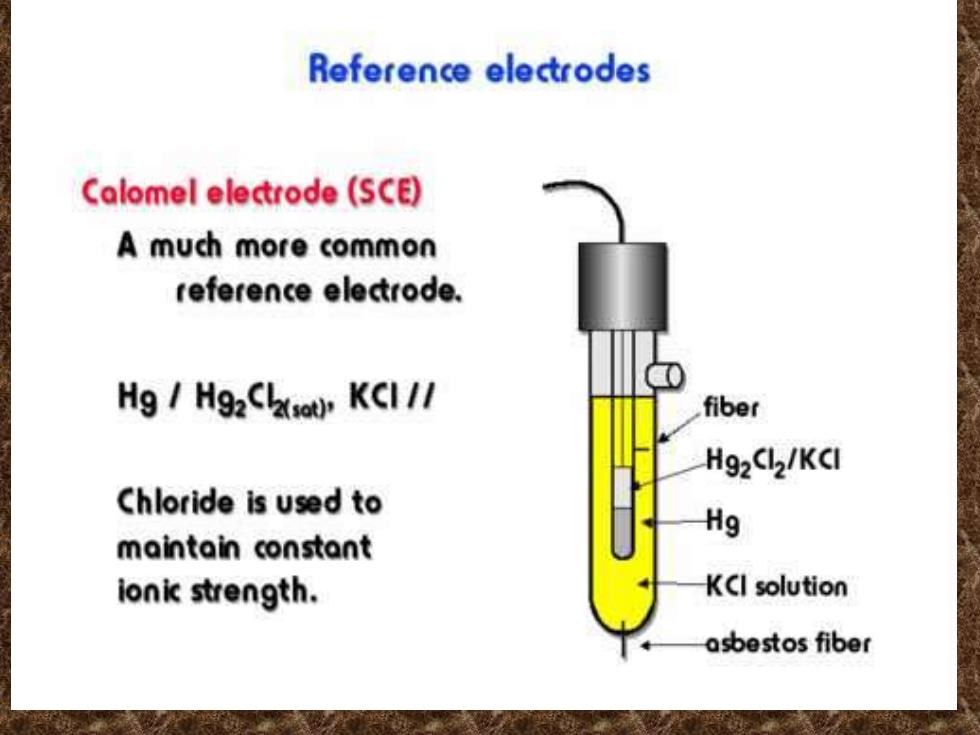
Reference electrodes Calomel electrode (SCE) A much more common reference electrode. Hg Hg2C KCI// fiber Hg2Cl2/KCl Chloride is used to Hg maintain constant ionic strength. KCI solution asbestos fiber