
Tannin Chemistry Tannin Chemistry This site contains information on the plant secondary metabolites known as tannins.The Hagerman laboratory methods originally in the"Tannin Handbook" are now available through this site,linked to information about tannins.Hyperlinks are indicated by blue text.Turn on Acrobat bookmarks to see titles of the pages. Condensed Tannin Structural Chemistry .Hydrolyzable Tannin Structural Chemistry Purification and Identification Quantitative Analysis Biological Activities Biosynthesis The "Tannin Handbook"can no longer be obtained in hard copy.Instead,print the pages you need from this site. Send me e-mail at hagermae@muohio.edu Or write to me at Professor Ann E.Hagerman Department of Chemistry and Biochemistry Miami University Oxford,OH 45056 USA am1998.2002.Th material may be copied for use within a single laboratory but cannot be copied for distribution o
Tannin Chemistry Tannin Chemistry This site contains information on the plant secondary metabolites known as tannins. The Hagerman laboratory methods originally in the "Tannin Handbook" are now available through this site, linked to information about tannins. Hyperlinks are indicated by blue text. Turn on Acrobat bookmarks to see titles of the pages. • Condensed Tannin Structural Chemistry • Hydrolyzable Tannin Structural Chemistry • Purification and Identification • Quantitative Analysis • Biological Activities • Biosynthesis The "Tannin Handbook" can no longer be obtained in hard copy. Instead, print the pages you need from this site. Send me e-mail at hagermae@muohio.edu Or write to me at Professor Ann E. Hagerman Department of Chemistry and Biochemistry Miami University Oxford, OH 45056 USA © Ann E. Hagerman 1998, 2002. This material may be copied for use within a single laboratory but cannot be copied for distribution or publication without permission of the author
What is a tannin? WHAT IS A TANNIN? Plants accumulate a wide variety of"secondary"compounds,including alkaloids,terpenes and phenolics Although these compounds apparently do not function in "primary"metabolism such as biosynthesis, biodegradation and other energy conversions of intermediary metabolism,they do have diverse biological activities ranging from toxicity to hormonal mimicry,and may play a role in protecting plants from herbivory and disease Phenolic metabolism in plants is complex,and yields a wide array of compounds ranging from the familiar flower pigments(anthocyanidins)to the complex phenolics of the plant cell wall(lignin). However,the group of phenolic compounds known as tannins is clearly distinguished from other plant secondary phenolics in their chemical reactivities biological activities. Tradition use of tannins as agents for converting animal hides to leather("tanning")is one manifestation of the most obvious activity of the tannins:their ability to interact with and precipitate proteins,including the proteins found in animal skin.The term"tannin"comes from the ancient Celtic word for oak,a typical source for tannins for leather making Bate-Smith defined tannins as "water-soluble phenolic compounds having molecular weights between 500 and 3000...[giving]the usual phenolic reactions...[and having]special properties such as the ability to precipiate alkaloids,gelatin and other proteins" Haslam has more recently substituted the term"polyphenol"for"tannin",in an attempt to emphasize the multiplicity of phenolic groups characteristic of these compounds.He notes that molecular weights as high as 20,000 have been reported,and that tannins complex not only with proteins and alkaloids but also with certain polysaccharides.I prefer to use the term tannin,which emphasizes the character which sets tannins apart from all other phenolics:the ability to precipitate proteins. Ann E.Hagerman 1998,2002.This material may be copied for use within a single laboratory but cannot be copied for distribution or publication without permission of the author Return to Tannin Chemistry Home Page
What is a tannin? WHAT IS A TANNIN? Plants accumulate a wide variety of "secondary" compounds, including alkaloids, terpenes and phenolics. Although these compounds apparently do not function in "primary" metabolism such as biosynthesis, biodegradation and other energy conversions of intermediary metabolism, they do have diverse biological activities ranging from toxicity to hormonal mimicry, and may play a role in protecting plants from herbivory and disease. Phenolic metabolism in plants is complex, and yields a wide array of compounds ranging from the familiar flower pigments (anthocyanidins) to the complex phenolics of the plant cell wall (lignin). However, the group of phenolic compounds known as tannins is clearly distinguished from other plant secondary phenolics in their chemical reactivities & biological activities. Tradition use of tannins as agents for converting animal hides to leather ("tanning") is one manifestation of the most obvious activity of the tannins: their ability to interact with and precipitate proteins, including the proteins found in animal skin. The term "tannin" comes from the ancient Celtic word for oak, a typical source for tannins for leather making. Bate-Smith defined tannins as "water-soluble phenolic compounds having molecular weights between 500 and 3000...[giving] the usual phenolic reactions...[and having] special properties such as the ability to precipiate alkaloids, gelatin and other proteins". Haslam has more recently substituted the term "polyphenol" for "tannin", in an attempt to emphasize the multiplicity of phenolic groups characteristic of these compounds. He notes that molecular weights as high as 20,000 have been reported, and that tannins complex not only with proteins and alkaloids but also with certain polysaccharides. I prefer to use the term tannin, which emphasizes the character which sets tannins apart from all other phenolics: the ability to precipitate proteins. © Ann E. Hagerman 1998, 2002. This material may be copied for use within a single laboratory but cannot be copied for distribution or publication without permission of the author. Return to Tannin Chemistry Home Page
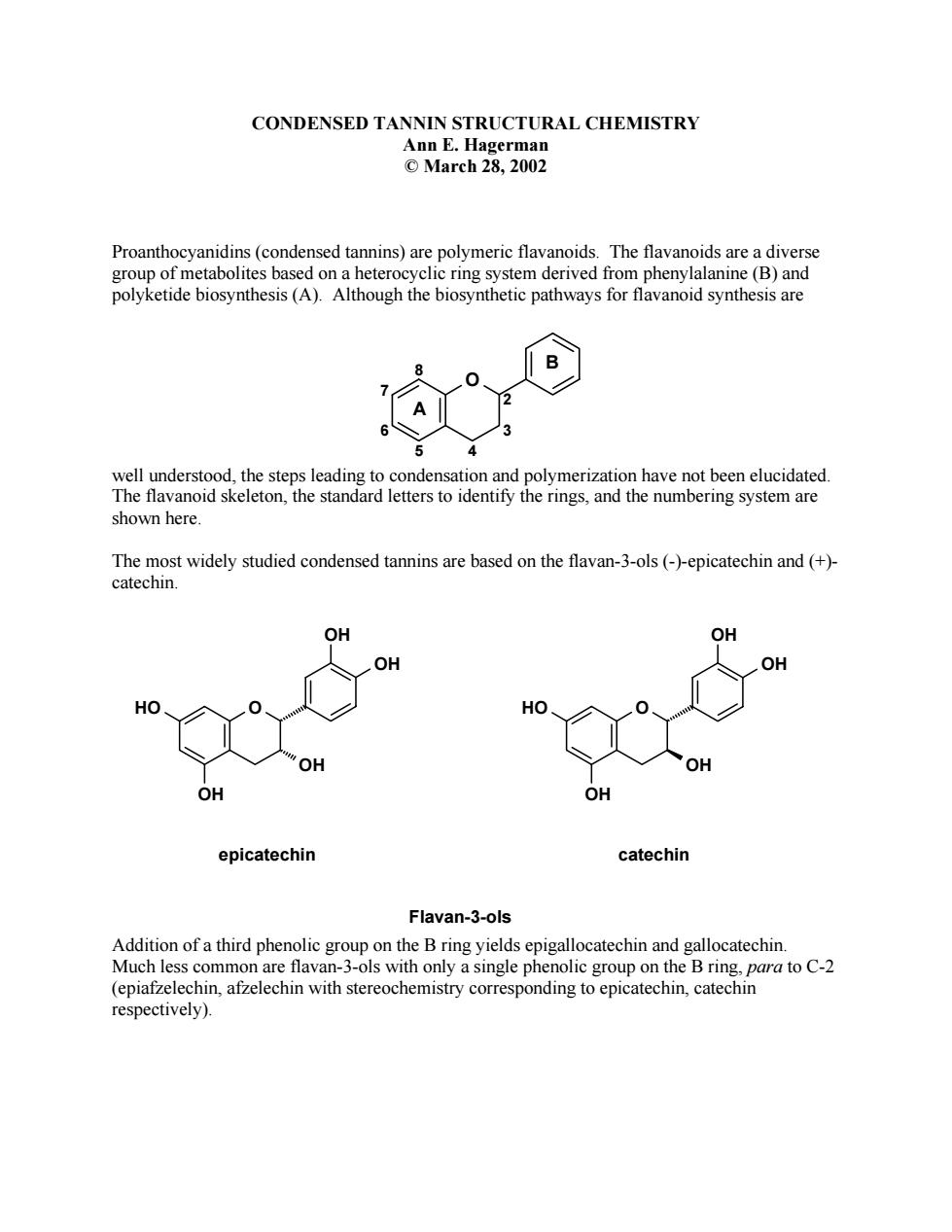
CONDENSED TANNIN STRUCTURAL CHEMISTRY Ann E.Hagerman ©1arch28.2002 Proanthocyanidins(condensed tannins)are polymeric flavanoids.The flavanoids are a diverse group of metabolites based on a heterocyclic ring system derived from phenylalanine(B)and polyketide biosynthesis(A).Although the biosynthetic pathways for flavanoid synthesis are well understood,the steps leadin condensation and polymerization have not been elucidated The flavanoid skeleton he standard letters to identify the r and the numbering system are shown here The mo t widely studied cond on the fla van-3-ols (-)-epicat and (+ epicatechin catechin Flavan-3-ols Addition of a third phenolic group on the B ring yields epigallocatechin and gallocatechin Much less c common are flav an-3-ols with only ngle phenolic grov on the B ring. respectively)
CONDENSED TANNIN STRUCTURAL CHEMISTRY Ann E. Hagerman © March 28, 2002 Proanthocyanidins (condensed tannins) are polymeric flavanoids. The flavanoids are a diverse group of metabolites based on a heterocyclic ring system derived from phenylalanine (B) and polyketide biosynthesis (A). Although the biosynthetic pathways for flavanoid synthesis are well understood, the steps leading to condensation and polymerization have not been elucidated. The flavanoid skeleton, the standard letters to identify the rings, and the numbering system are shown here. The most widely studied condensed tannins are based on the flavan-3-ols (-)-epicatechin and (+)- catechin. Addition of a third phenolic group on the B ring yields epigallocatechin and gallocatechin. Much less common are flavan-3-ols with only a single phenolic group on the B ring, para to C-2 (epiafzelechin, afzelechin with stereochemistry corresponding to epicatechin, catechin respectively). 7 6 5 8 4 3 2 O A B HO O OH OH OH OH epicatechin HO O OH OH OH OH catechin Flavan-3-ols
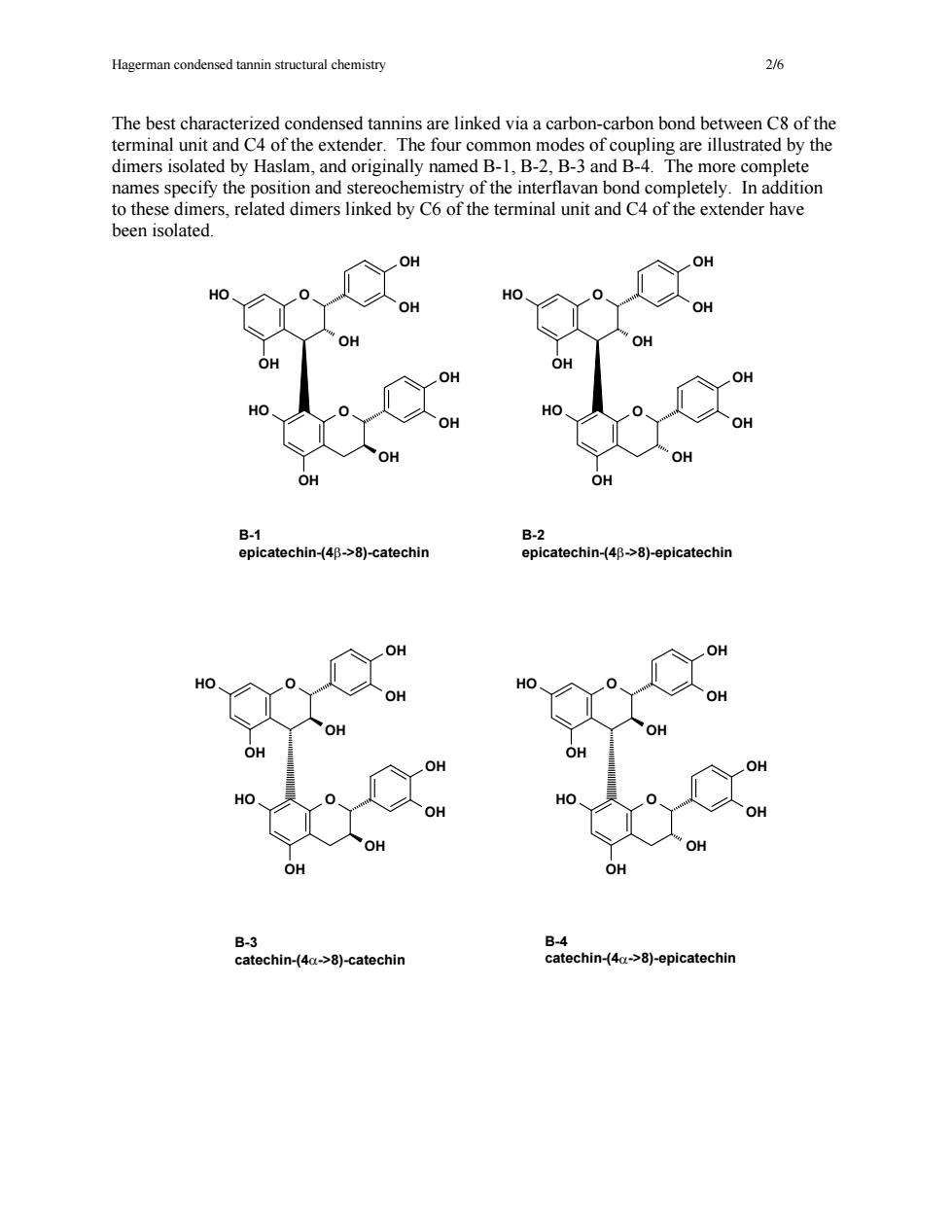
Hagerman stuctural hemistr The best characterized condensed tannins are linked via a carbon-carbon bond between C8 of the terminal unit and C4 of the extender.The four common modes of coupling are illustrated by the dimers isolated by Haslam,and originally named B-1,B-2,B-3 and B-4.The more complete names specify the position and stereochemistry of the interflavan bond completely.In addition to these dimers,related dimers linked by C6 of the terminal unit and C4 of the extender have been isolated. OH B-2 epicatechin-(4B->8)-catechin epicatechin-(40->8)-epicatechin B-3 catechin-(4a->8)-catechin chin-4a>8)-epicatechir
Hagerman condensed tannin structural chemistry 2/6 The best characterized condensed tannins are linked via a carbon-carbon bond between C8 of the terminal unit and C4 of the extender. The four common modes of coupling are illustrated by the dimers isolated by Haslam, and originally named B-1, B-2, B-3 and B-4. The more complete names specify the position and stereochemistry of the interflavan bond completely. In addition to these dimers, related dimers linked by C6 of the terminal unit and C4 of the extender have been isolated. HO O OH OH OH OH HO O OH OH OH OH HO O OH OH OH OH HO O OH OH OH OH B-3 catechin-(4α->8)-catechin B-4 catechin-(4α->8)-epicatechin HO O OH OH OH OH HO O OH OH OH OH HO O OH OH OH OH HO O OH OH OH OH B-1 epicatechin-(4β->8)-catechin B-2 epicatechin-(4β->8)-epicatechin
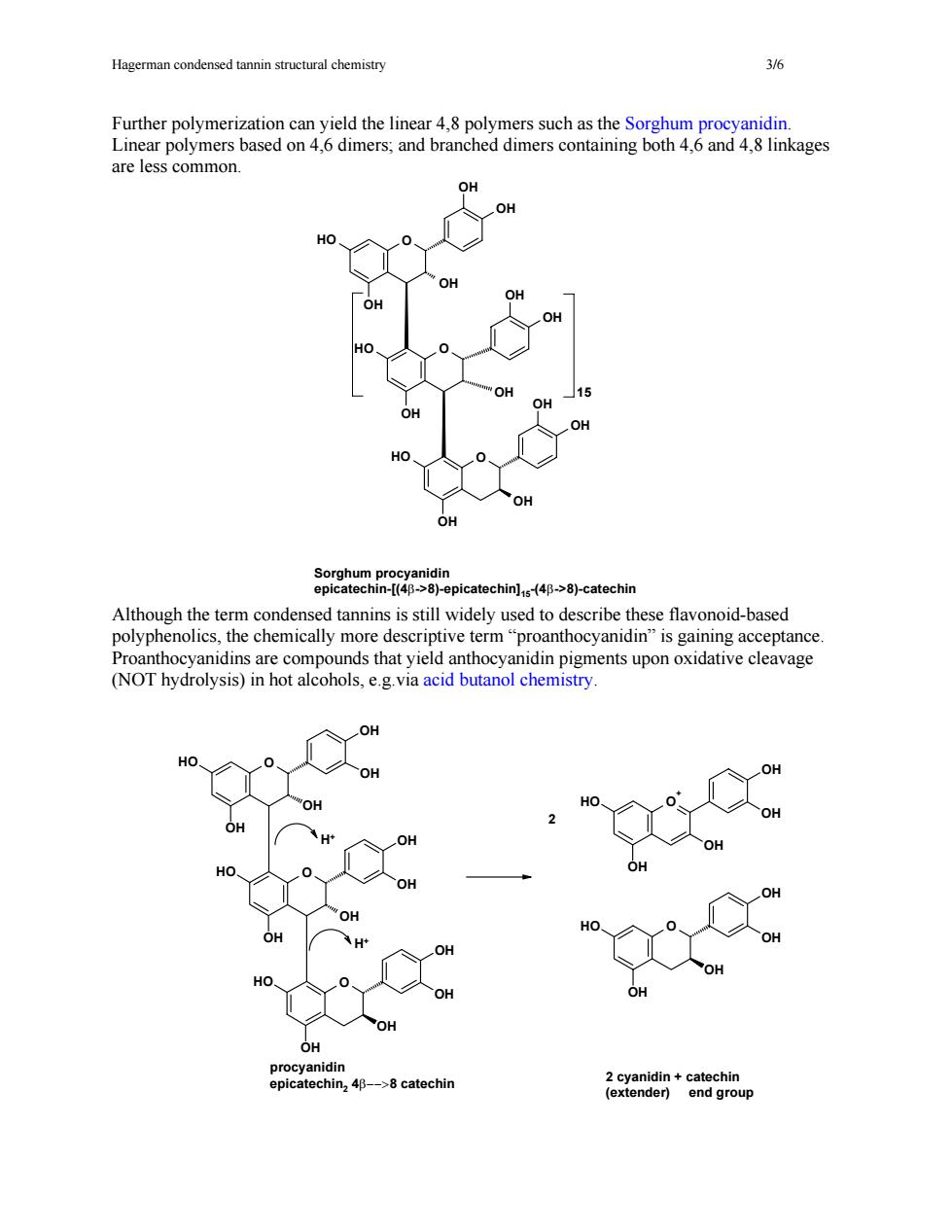
Hagerman condensed tannin structural chemistry Further polymerization can yield the linear 4.8 polymers such as the Sorghum procyanidin. Linear polymers based on 4,6 dimers;and branched dimers containing both 4,6 and 4,8 linkages are less common picatechin-catchin Although the term conde sed tannin is still widely used to describe these flavonoid-based polyphenolics,the chemically more descriptive term"proanthocyanidin"is gaining acceptance Proanthocyanidins are compounds that yield anthocyanidin pigments upon oxidative cleavage (NOT hydrolysis)in hot alcohols,e.g.via acid butanol chemistry. OH in2 46->8 catechir a2ena8n+eng8gop
Hagerman condensed tannin structural chemistry 3/6 Further polymerization can yield the linear 4,8 polymers such as the Sorghum procyanidin. Linear polymers based on 4,6 dimers; and branched dimers containing both 4,6 and 4,8 linkages are less common. Although the term condensed tannins is still widely used to describe these flavonoid-based polyphenolics, the chemically more descriptive term “proanthocyanidin” (NOT hydrolysis) in hot alcohols, e.g.via acid butanol chemistry. is gaining acceptance. Proanthocyanidins are compounds that yield anthocyanidin pigments upon oxidative cleavage HO O OH OH OH OH O O O OH OH OH HO OH OH OH HO OH OH OH OH OH OH HO O+ OH OH HO OH OH procyanidin epicatechin2 4β−−>8 catechin 2 cyanidin + catechin (extender) end group H+ H+ 2 O O OH HO HO OH OH OH OH HO O OH OH OH OH OH OH OH 15 Sorghum procyanidin epicatechin-[(4β->8)-epicatechin]15-(4β->8)-catechin