
■l.Halogenation +Br2 hv B HBr anhy.AICla Highly selective:bromination of 3 C CH3 CH3 CH3- C-H Br2 hv> CH3一C-Br+ HBr CH3 CH3 90%
Highly selective: bromination of 3 C 90% CH3 C + HBr CH3 CH3 Br h CH3 C + Br2 CH3 CH3 H RH + Cl2 RCl + HCl u.v. Cl Cl 2 anhy. AlCl3 1. Halogenation + HBr H Br h + Br2 H H

清我8米大研 Sec 1 Preparation of RX Free radical halogenation produces mixtures,not good lab synthesis ounless:all H's are equivalent,or ohalogenation is highly selective. Free radical allylic halogenation oproduces alkyl halide with double bond on the neighboring carbon
Sec 1 Preparation of RX Free radical halogenation produces mixtures, not good lab synthesis unless: all H’s are equivalent, or halogenation is highly selective. Free radical allylic halogenation produces alkyl halide with double bond on the neighboring carbon
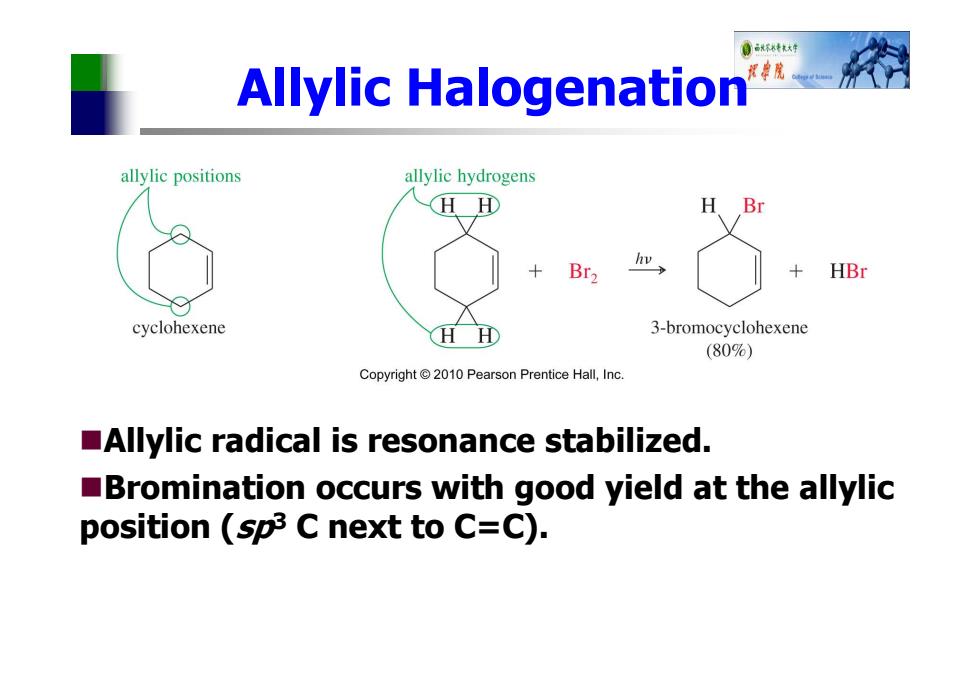
0环行 Allylic Halogenation 摆院 allylic positions allylic hydrogens HH H Br Br2 hv HBr cyclohexene HH 3-bromocyclohexene (80%) Copyright 2010 Pearson Prentice Hall,Inc. Allylic radical is resonance stabilized. Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
Allylic Halogenation Allylic radical is resonance stabilized. Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
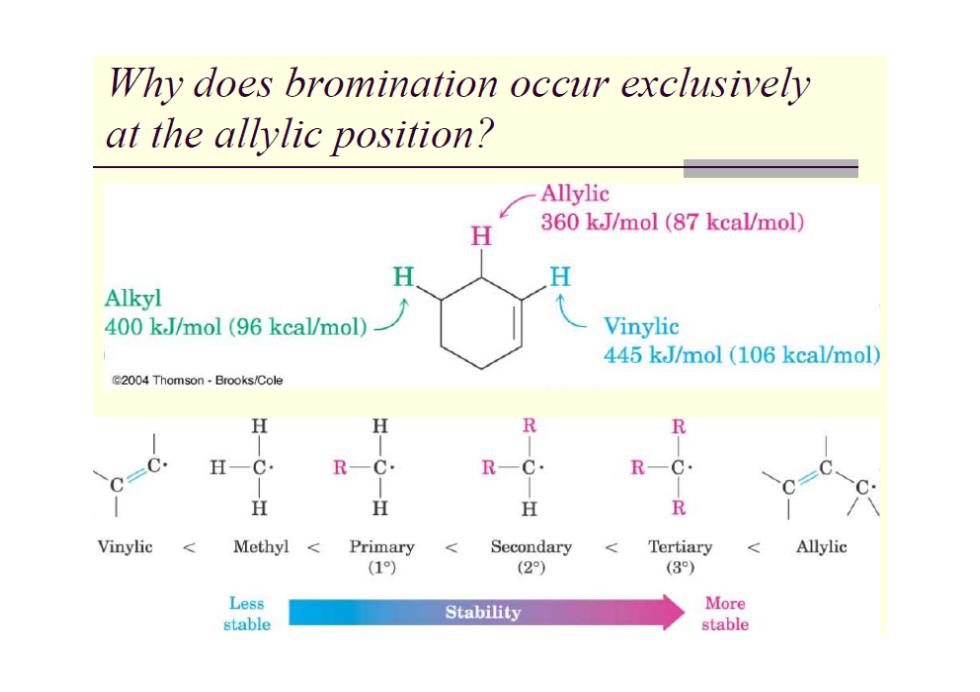
Why does bromination occur exclusively at the allylic position? Allylic H 360 kJ/mol(87 kcal/mol) H H Alkyl 400 kJ/mol (96 kcal/mol) Vinylic 445 kJ/mol (106 kcal/mol) 62004 Thomson Brooks/Cole R R H一C R-C. R—C R-C. H H H R Vinylic Methyl Primary Secondary Tertiary <Allylic (1) (2) (3) Less Stability More stable stable

Allylic Halogenation Avoid a large excess of Br>by using N-bromosuccinimide (NBS)to generate Br2 as product HBr is formed. N-bromosuccinimide (NBS)is an allylic brominating agent. Keeps the concentration of Br2 low. Br
Allylic Halogenation Avoid a large excess of Br 2 by using N-bromosuccinimide (NBS) to generate Br 2 as product HBr is formed. N-bromosuccinimide (NBS) is an allylic brominating agent. Keeps the concentration of Br 2 low. N O O Br + HBr N O O H + Br 2