
1.滴定开始前溶液中氯离子浓度为溶液的原始浓度[Cl-] =0.1000mol/LpCl =-lgl.000×10-1 = 1.002.滴定至化学计量点前加入AgNO,溶液18.00ml时,溶液中TCI-浓度为0.1000 ×2.00° =5.26×10-3 pCl=2.279[CI-20.00 +18.00而Ag+浓度则因为[Ag+][C1-] =Ksp =1.56×10-10pAg + pCl = -lgKsp = 9.74故pAg = 9.74 - 2.28 = 7.46同理,当加入AgNO,溶液19.98ml时,溶液中剩余的CI-浓度为 : [CI-]= 5.0 ×10-5pCI= 4.30pAg = 5.44
1. 滴定开始前 溶液中氯离子浓度为溶液的原始浓度 [Cl-]= 0.1000 mol/L pCl = -lgl.000×10-1 = l.00 2. 滴定至化学计量点前 加入 AgNO3溶液 18.00ml时,溶液中 Cl-浓度为: 而Ag+浓度则因为 [Ag+ ][Cl- ] = Ksp = 1.56×10-10 pAg+pCl = -lgKsp = 9.74 故 pAg = 9.74 - 2.28 = 7.46 同理,当加入AgNO3溶液 19.98ml时,溶液中剩余的Cl-浓度 为:[Cl- ]= 5.0 ×10-5 pCl = 4.30 pAg = 5.44 5.26 10 pCl 2.279 20.00 18.00 0.1000 2.00 [Cl ] 3 = = + = − −

3.化学计量点时溶液是AgC的饱和溶液pAg =pCl=pKsp/2= 4.874.化学计量点后[Ag]浓度由过量的AgNO3浓度决定,当滴入AgNO3溶液20.02ml时(过量AgNO30.02ml时则[Ag+] =5.0×10-5mol / L。pAg = 4.30pCl = 9.74- 4.30 = 5.44
3. 化学计量点时 溶液是AgCl的饱和溶液 pAg = pCl = pKsp/2= 4.87 4. 化学计量点后 [Ag+ ]浓度由过量的AgNO3浓度决 定,当滴入 AgNO3溶液 20.02ml时(过量 AgNO3 0.02ml时 则[Ag+]= 5.0×10-5 mol/L。 pAg = 4.30 pCl = 9.74- 4.30 = 5.44

以0.1000mol/LAgNO3溶液滴定20.00ml0.1000mol/LNaCl或表 8-10.1000mol/LKBr溶液时等当点前后Ag与pX的变化滴定 Br滴定CI-加入0.1mol/LAgNO溶液量%pBrpAgmlpClpAg01.01.00.007. 52.310.0902.318.006. 83.09.33.019.60983.39.06.519.80993.34.08.35.899.84.019.968.05. 54.399.914.319.986.156.154. 91004.920.008.04.35.54.3100.120.028.34.05.84. 020.04100.23.39.01016. 53.320.203.09.36.83. 020.401022.57. 52.310.022.00110
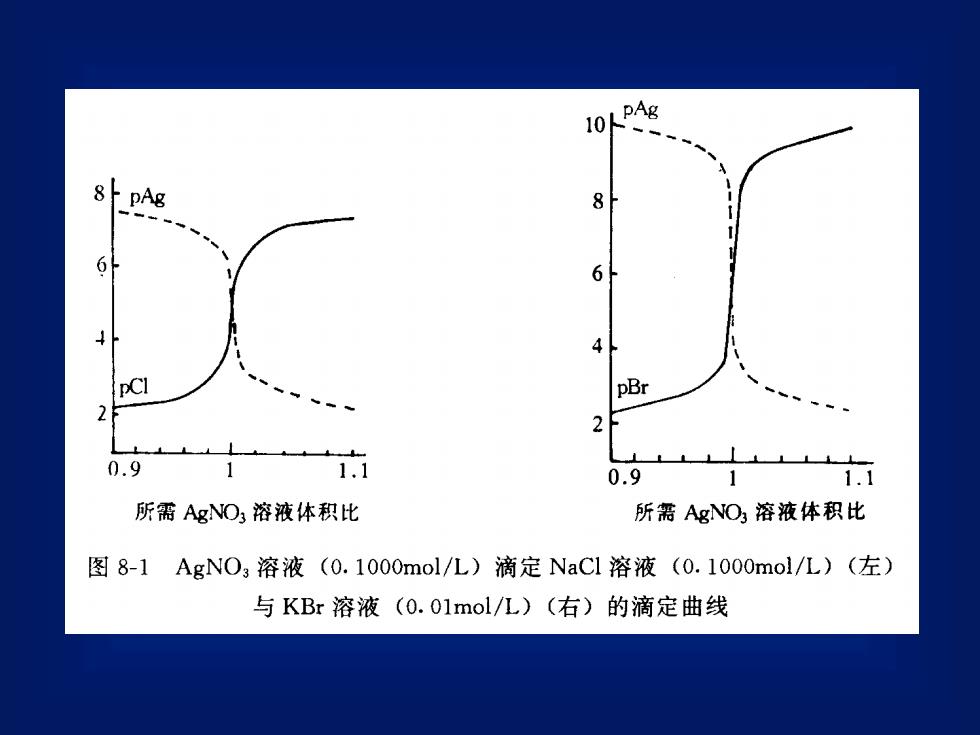
LpAg108/ pAg86644pClpBr20.91.110.911.1所需AgNO溶液体积比所需AgNO,溶液体积比图8-1AgNO,溶液(0.1000mol/L)滴定NaCl溶液(0.1000mo1/L)(左)与KBr溶液(0.01mol/L)(右)的滴定曲线
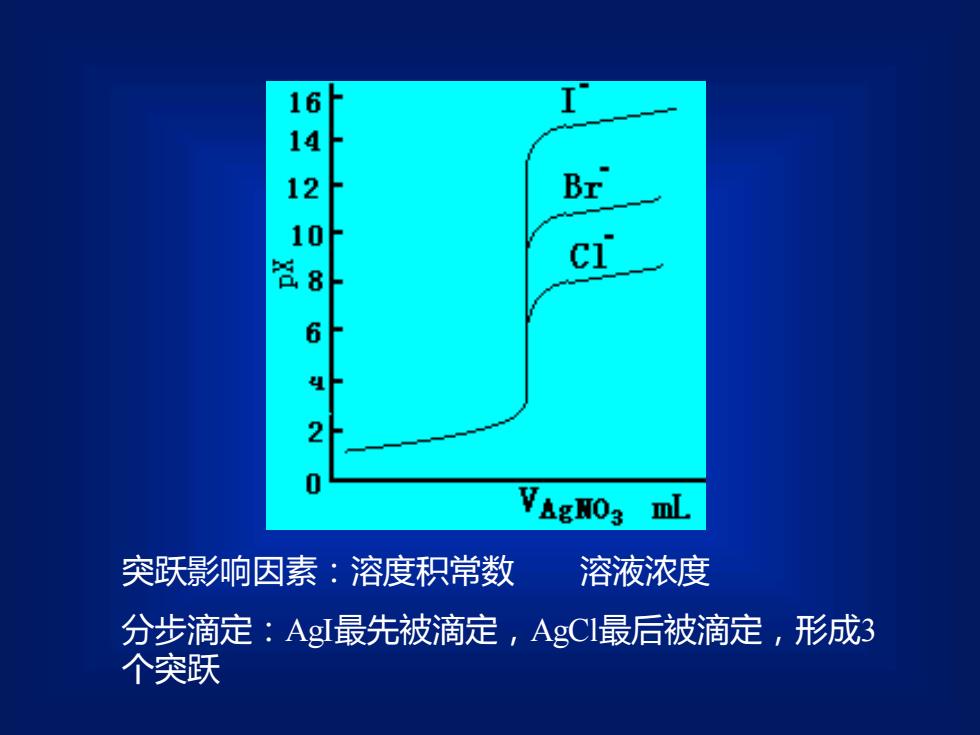
160238d62VAgH03ML溶液浓度突跃影响因素:溶度积常数分步滴定:AgI最先被滴定,AgCl最后被滴定,形成3个突跃
突跃影响因素:溶度积常数 溶液浓度 分步滴定:AgI最先被滴定,AgCl最后被滴定,形成3 个突跃