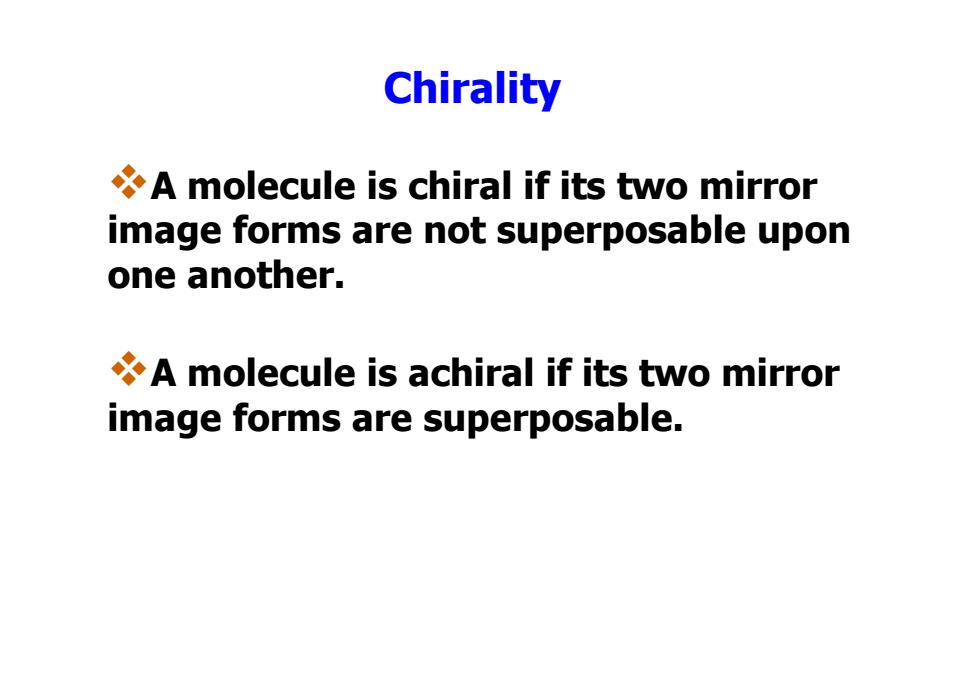
Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable
Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable
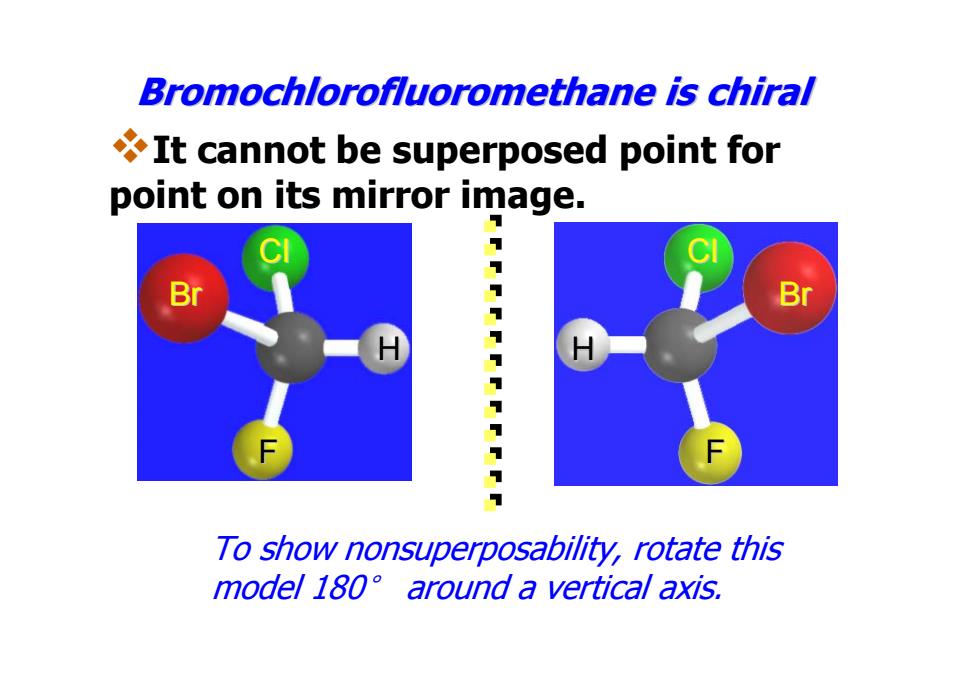
Bromochlorofluoromethane is chiral It cannot be superposed point for point on its mirror image. B Br 7 111111T To show nonsuperposability,rotate this model 180 around a vertical axis
Br Cl H F Bromochlorofluoromethane Bromochlorofluoromethane is chiral H Cl Br F To show nonsuperposability, rotate this model 180° around a vertical axis. It cannot be superposed point for point on its mirror image
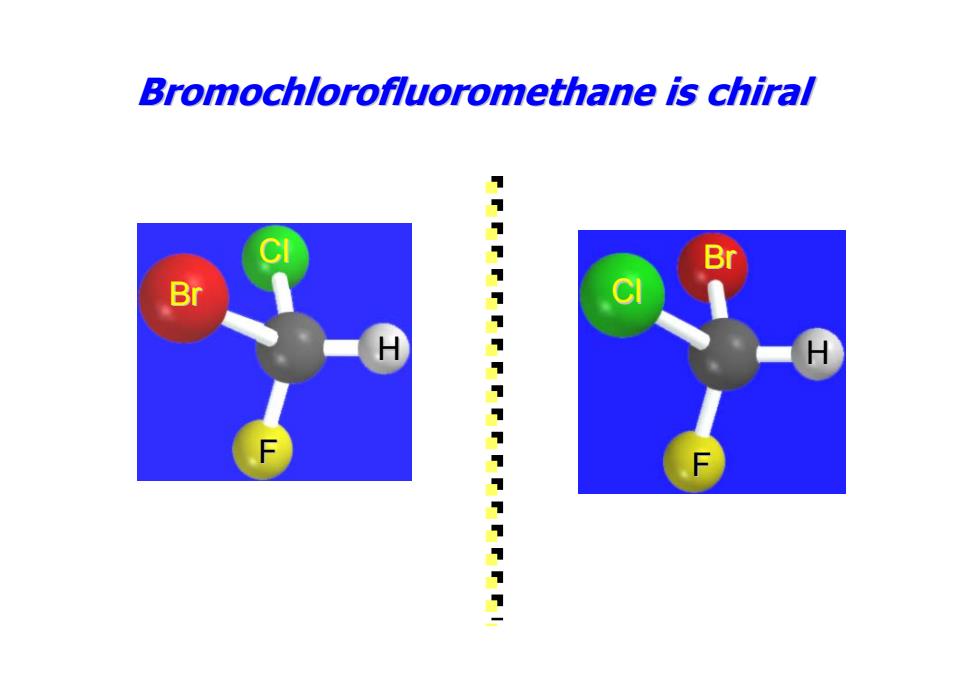
Bromochlorofluoromethane is chiral Br 1111111111111111111
Br Cl H F Bromochlorofluoromethane Bromochlorofluoromethane is chiral H Cl Br F
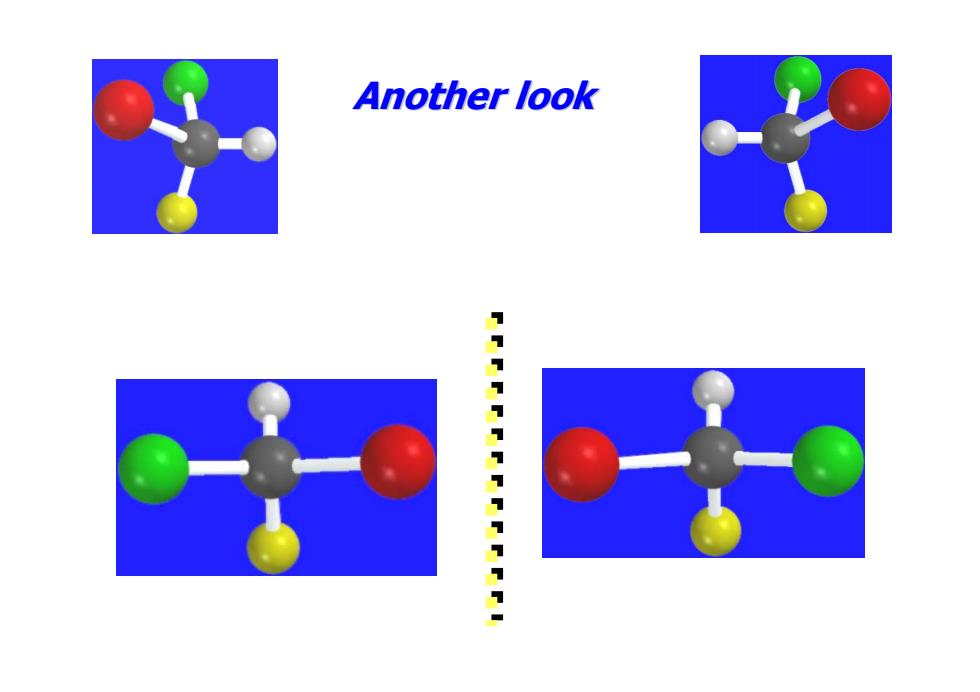
Another look 1111111711111
Another look Another look
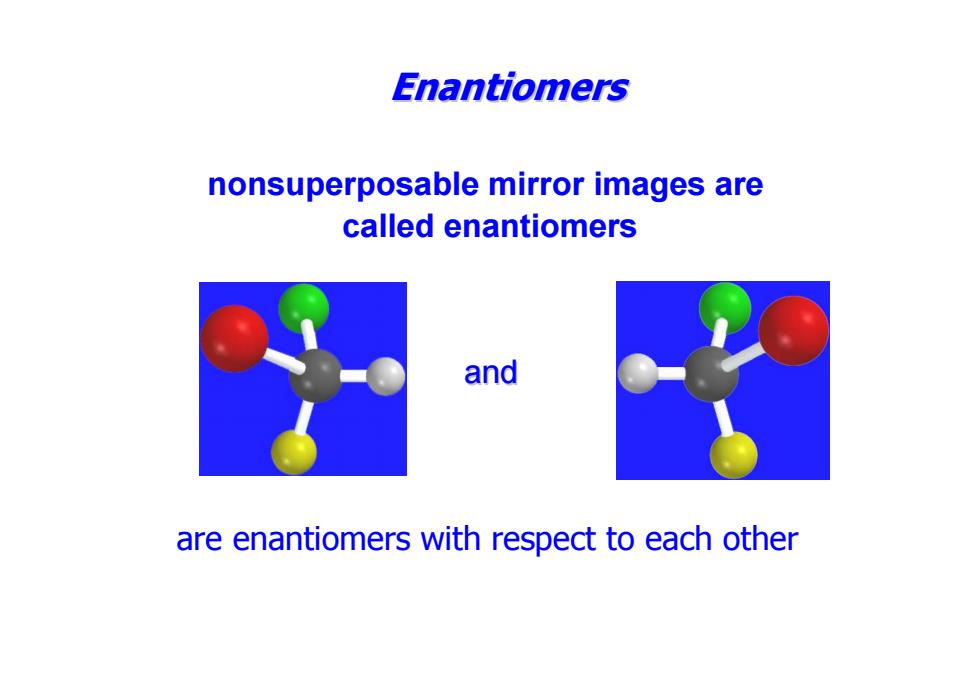
Enantiomers nonsuperposable mirror images are called enantiomers and are enantiomers with respect to each other
are enantiomers with respect to each other and nonsuperposable mirror images are called enantiomers Enantiomers Enantiomers