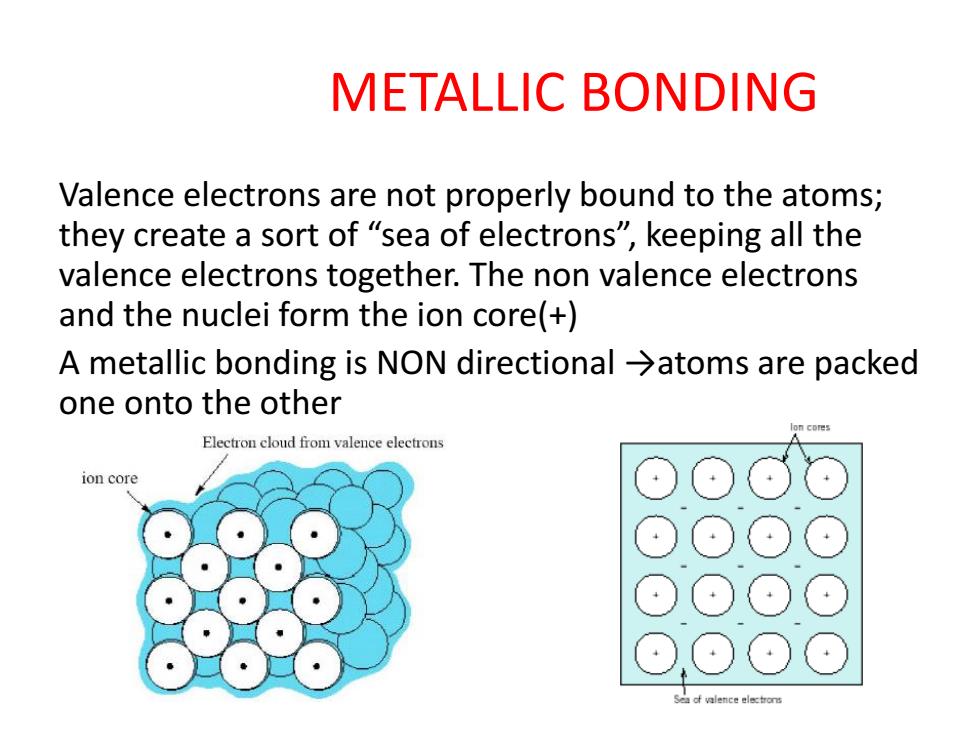
METALLIC BONDING Valence electrons are not properly bound to the atoms; they create a sort of "sea of electrons",keeping all the valence electrons together.The non valence electrons and the nuclei form the ion core(+) A metallic bonding is NON directional >atoms are packed one onto the other lon cores Electron cloud from valence electrons ion core Sea of valence electrons
(c)METALLIC BONDING Valence electrons are not properly bound to the atoms; they create a sort of “sea of electrons”, keeping all the valence electrons together. The non valence electrons and the nuclei form the ion core(+) A metallic bonding is NON directional →atoms are packed one onto the other
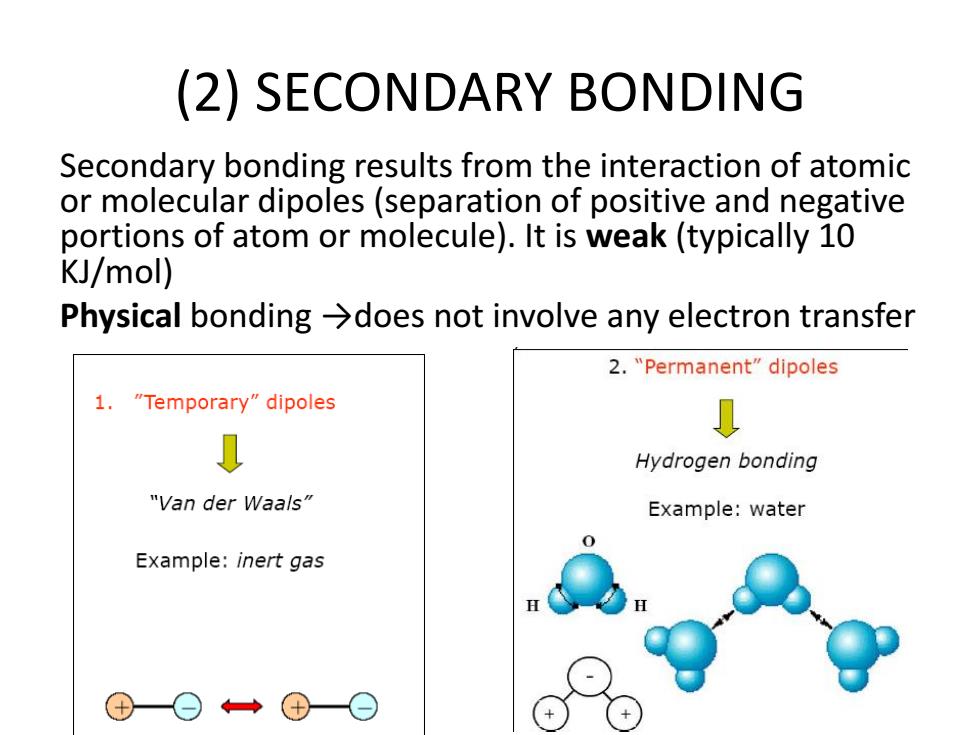
(2)SECONDARY BONDING Secondary bonding results from the interaction of atomic or molecular dipoles(separation of positive and negative portions of atom or molecule).It is weak(typically 10 KJ/mol) Physical bonding >does not involve any electron transfer 2."Permanent"dipoles 1."Temporary"dipoles Hydrogen bonding "Van der Waals" Example:water 0 Example:inert gas
(2) SECONDARY BONDING Secondary bonding results from the interaction of atomic or molecular dipoles (separation of positive and negative portions of atom or molecule). It is weak (typically 10 KJ/mol) Physical bonding →does not involve any electron transfer
2.2.Classes of materials interesting under an engineering approach (1)-METALS (2)-CERAMICS (3)-GLASSES (4)-POLYMERS (5)-COMPOSITE
2.2. Classes of materials interesting under an engineering approach (1)- METALS (2)- CERAMICS (3)- GLASSES (4)- POLYMERS (5)- COMPOSITE
(1)-Metals(I】 Main Characteristics 。Opaque Reflect light High mechanical strength 。Stiff Ductile Heat and electricity conductors Resistant to thermal shock Subject to corrosion and oxidation ·Easy to be machined
(1)-Metals(Ⅰ) Main Characteristics • Opaque • Reflect light • High mechanical strength • Stiff • Ductile • Heat and electricity conductors • Resistant to thermal shock • Subject to corrosion and oxidation • Easy to be machined
(1)-Metals(II) Made up of elements(Fe,Al,Mg,Cu,...) Typically in crystalline state Almost all in solid state at room temperature Originate alloys Example:steels Aluminum alloys,titanium
(1)-Metals(Ⅱ) Made up of elements(Fe, Al, Mg, Cu,…) Typically in crystalline state Almost all in solid state at room temperature Originate alloys Example: steels Aluminum alloys , titanium, …