
2013-3-6 Chapter 5 The Second Law of Thermodynamics Learning Outcomes Demonstrate understanding of key concepts related to the second law of thermodynamics,including alternative statements of the second law,the internally reversible process,and the Kelvin temperature scale. List several important irreversibilities. 1
2013-3-6 1 Chapter 5 The Second Law of Thermodynamics Learning Outcomes ►Demonstrate understanding of key concepts related to the second law of thermodynamics, including alternative statements of the second law, the internally reversible process, and the Kelvin temperature scale. ►List several important irreversibilities

2013-3-6 Learning Outcomes,cont. Assess the performance of power cycles and refrigeration and heat pump cycles using,as appropriate,the corollaries of Secs.5.6.2 and 5.7.2,together with Egs 5.9-5.11. Describe the Carnot cycle. Interpret the Clausius inequality as expressed by Eq.5.13. Aspects of the Second Law of Thermodynamics From conservation of mass and energy principles,mass and energy cannot be created or destroyed. For a process,conservation of mass and energy principles indicate the disposition of mass and energy but do not infer whether the process can actually occur. The second law of thermodynamics provides the guiding principle for whether a process can occur. 2
2013-3-6 2 Learning Outcomes, cont. ►Assess the performance of power cycles and refrigeration and heat pump cycles using, as appropriate, the corollaries of Secs. 5.6.2 and 5.7.2, together with Eqs. 5.9-5.11. ►Describe the Carnot cycle. ►Interpret the Clausius inequality as expressed by Eq. 5.13. Aspects of the Second Law of Thermodynamics ►From conservation of mass and energy principles, mass and energy cannot be created or destroyed. ►For a process, conservation of mass and energy principles indicate the disposition of mass and energy but do not infer whether the process can actually occur. ►The second law of thermodynamics provides the guiding principle for whether a process can occur

2013-3-6 Aspects of the Second Law of Thermodynamics The second law of thermodynamics has many aspects,which at first may appear different in kind from those of conservation of mass and energy principles.Among these aspects are: predicting the direction of processes establishing conditions for equilibrium determining the best theoretical performance of cycles,engines,and other devices. level. Aspects of the Second Law of Thermodynamics Other aspects of the second law include: defining a temperature scale independent of the properties of any thermometric substance. developing means for evaluating properties such as u and h in terms of properties that are more readily obtained experimentally. Scientists and engineers have found additional uses of the second law and deductions from itit also has been used in philosophy,economics,and other disciplines far removed from engineering thermodvnamics. 3
2013-3-6 3 Aspects of the Second Law of Thermodynamics ►predicting the direction of processes. ►establishing conditions for equilibrium. ►determining the best theoretical performance of cycles, engines, and other devices. ►evaluating quantitatively the factors that preclude attainment of the best theoretical performance level. The second law of thermodynamics has many aspects, which at first may appear different in kind from those of conservation of mass and energy principles. Among these aspects are: Aspects of the Second Law of Thermodynamics ►defining a temperature scale independent of the properties of any thermometric substance. ►developing means for evaluating properties such as u and h in terms of properties that are more readily obtained experimentally. Scientists and engineers have found additional uses of the second law and deductions from it. It also has been used in philosophy, economics, and other disciplines far removed from engineering thermodynamics. Other aspects of the second law include:
2013-3-6 Second Law of Thermodynamics Alternative Statements There is no simple statement that captures all aspects of the second law.Several alternative formulations of the second law are found in the technical literature.Three prominent ones are: Clausius Statement Kelvin-Planck Statement Entropy Statement Second Law of Thermodynamics Alternative Statements The focus of Chapter 5 is on the Clausius and Kelvin-Planck statements. The Entropy statement is developed and applied in Chapter 6. Like every physical law,the basis of the second law of thermodynamics is experimental evidence.While the three forms given are not directly demonstrable in the laboratory, deductions from them can be verified experimentally,and this infers the validity of the second law statements. 4
2013-3-6 4 Second Law of Thermodynamics Alternative Statements ►Clausius Statement ►Kelvin-Planck Statement ►Entropy Statement There is no simple statement that captures all aspects of the second law. Several alternative formulations of the second law are found in the technical literature. Three prominent ones are: Second Law of Thermodynamics Alternative Statements ►The focus of Chapter 5 is on the Clausius and Kelvin-Planck statements. ►The Entropy statement is developed and applied in Chapter 6. ►Like every physical law, the basis of the second law of thermodynamics is experimental evidence. While the three forms given are not directly demonstrable in the laboratory, deductions from them can be verified experimentally, and this infers the validity of the second law statements
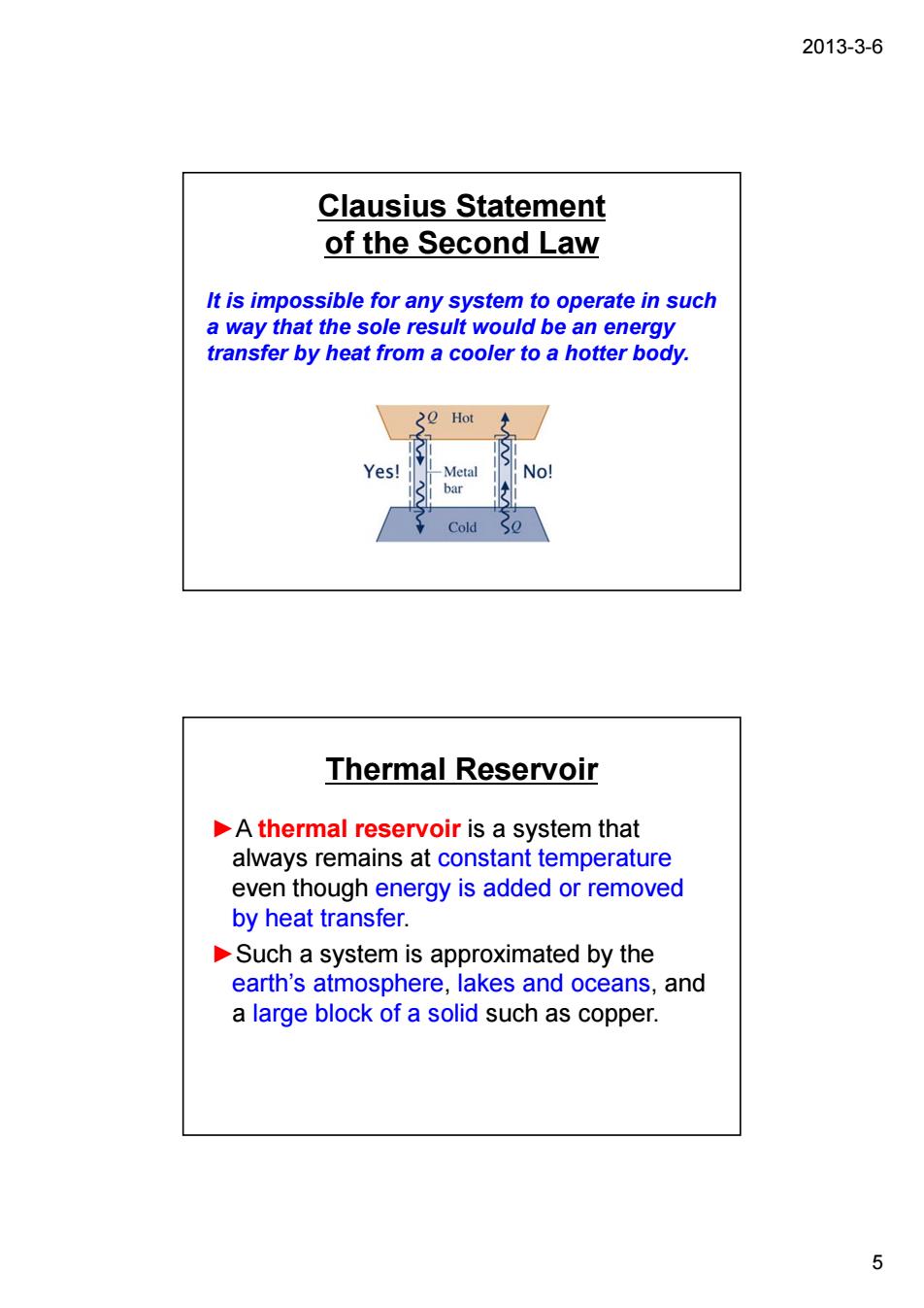
2013-3-6 Clausius Statement of the Second Law It is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter body. 20 Hot Thermal Reservoir A thermal reservoir is a system that always remains at constant temperature even though energy is added or removed by heat transfer. Such a system is approximated by the earth's atmosphere,lakes and oceans,and a large block of a solid such as copper. 5
2013-3-6 5 Clausius Statement of the Second Law It is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter body. Thermal Reservoir ►A thermal reservoir is a system that always remains at constant temperature even though energy is added or removed by heat transfer. ►Such a system is approximated by the earth’s atmosphere, lakes and oceans, and a large block of a solid such as copper