
The Recombinant Protein Handbook Protein Amplification and Simple Purification Amersham 18-1142-75 Biosciences Edition AB
18-1142-75 Edition AB Protein Amplification and Simple Purification The Recombinant Protein Handbook

The Recombinant Protein Handbook Protein Amplification and Simple Purification
1 The Recombinant Protein Handbook Protein Amplification and Simple Purification

Contents Introduction 5 Symbols and abbreviations CHAPTER 1 6 Choice of ost for Choice of vec or Vectors for fusion proteins Choice of fusion tag. CHAPTER 2. shootine protein amplification CHAPTER 3. GST fusion proteins. 13 Amplification DeconoGsTfsonpoeins 12 Purification and detection troubleshooting. 203 Thrombin cleavage and purification 3 FactorXaceareandpu0tcaic ie protease CHAPTER 4. .41 (His).fusion proteins 41 Amplification detection troubes Tag removal by enzymatic cleavage CHAPTER 5. 59 Handling inclusion bodie RetoldingotsolubilizedTecoambinantpotei5 CHAPTER 6. .63 Harvesting and extraction of recombinant proteins .63 CHAPTER 7. 67 Buffer exchange and desalting of recombinant proteins CHAPTER 8. Simple purification of other recombinant proteins. .71 Ready to use affinity purification os 1
2 Contents Introduction . 5 Symbols and abbreviations . 5 CHAPTER 1 . 6 Choice of host for protein amplification . 6 Choice of vectors . 7 Vectors for non-fusion proteins . 7 Vectors for fusion proteins . 8 Choice of fusion tag . 8 CHAPTER 2 . 9 Protein amplification . 9 Sample extraction . 9 Troubleshooting protein amplification . 9 CHAPTER 3 . 13 GST fusion proteins . 13 Amplification . 13 Purification . 14 Detection of GST fusion proteins . 21 Purification and detection troubleshooting . 28 Tag removal by enzymatic cleavage . 30 PreScission Protease cleavage and purification . 31 Thrombin cleavage and purification . 35 Factor Xa cleavage and purification . 37 Removal of thrombin, Factor Xa or other serine proteases . 39 CHAPTER 4 . 41 (His)6 fusion proteins . 41 Amplification . 41 Purification . 41 Detection of (His)6 fusion proteins . 53 Purification and detection troubleshooting . 56 Tag removal by enzymatic cleavage . 58 CHAPTER 5 . 59 Handling inclusion bodies . 59 Solubilization of inclusion bodies . 59 Refolding of solubilized recombinant proteins . 60 CHAPTER 6 . 63 Harvesting and extraction of recombinant proteins . 63 CHAPTER 7 . 67 Buffer exchange and desalting of recombinant proteins . 67 CHAPTER 8 . 71 Simple purification of other recombinant proteins . 71 Ready to use affinity purification columns . 71 Making a specific purification column . 73 Purification . 75

CHAPTER 9. 77 Multi-step purification of recombinant proteins(fusion and non-fusion). Appendix 1. 86 Map of the GST fusion vectors showing reading frames and main features Glutathione S-transferase (GST) Appendix 2. .88 Amino acids table Appendix 3. 90 Protein conversion data Appendix 4. 90 Centrifuges,rotors and carriers for use with MicroPlex 24 % Appendix 5. .91 tics Characteristics,cleaning and storage of Chelating Sepharose Appendix 6. 93 Column packing and preparation Appendix 7. 95 Converting from linear flow (cm/hour)to volumetric flow rates (ml/min)and vice versa. Appendix 8. 96 Selection of purification equipment % Appendix 9. 97 s and stan onditions for purification techniques o Exchange 97 Hydrophobic n tion Chromatography (HIC) 101 Additional reading and reference material ,104 Orde ring infor 105
3 CHAPTER 9 . 77 Multi-step purification of recombinant proteins (fusion and non-fusion) . 77 Selection and combination of purification techniques . 78 Appendix 1 . 86 Map of the GST fusion vectors showing reading frames and main features . 86 Glutathione S-transferase (GST) . 87 Appendix 2 . 88 Amino acids table. 88 Appendix 3 . 90 Protein conversion data . 90 Appendix 4. . 90 Centrifuges, rotors and carriers for use with MicroPlex 24 . 90 Appendix 5 . 91 Characteristics, cleaning and storage of Glutathione Sepharose . 91 Characteristics, cleaning and storage of Chelating Sepharose . 92 Appendix 6 . 93 Column packing and preparation . 93 Appendix 7 . 95 Converting from linear flow (cm/hour) to volumetric flow rates (ml/min) and vice versa . 95 Appendix 8 . 96 Selection of purification equipment . 96 Appendix 9 . 97 Principles and standard conditions for purification techniques . 97 Affinity Chromatography (AC) . 97 Ion Exchange (IEX) . 97 Hydrophobic Interaction Chromatography (HIC) . 99 Gel Filtration (GF) Chromatography . 100 Reversed Phase Chromatography (RPC) . 101 Additional reading and reference material . 104 Ordering information . 105
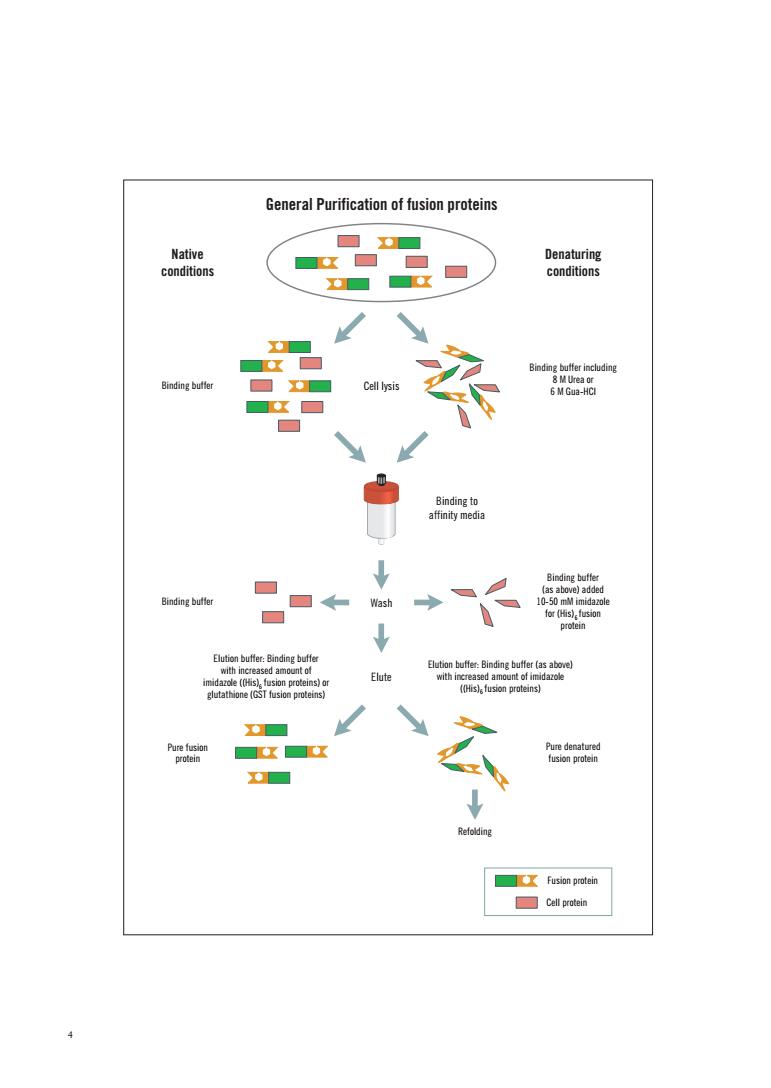
General Purification of fusion proteins Fesion protein
4 Native conditions Binding buffer Binding buffer Denaturing conditions Cell lysis Binding buffer including 8 M Urea or 6 M Gua-HCl Binding to affinity media Wash Elute Pure denatured fusion protein Pure fusion protein Refolding Binding buffer (as above) added 10-50 mM imidazole for (His) fusion protein 6 Elution buffer: Binding buffer (as above) with increased amount of imidazole ((His) fusion proteins) 6 Elution buffer: Binding buffer with increased amount of imidazole ((His) fusion proteins) or glutathione (GST fusion proteins) 6 General Purification of fusion proteins Fusion protein Cell protein