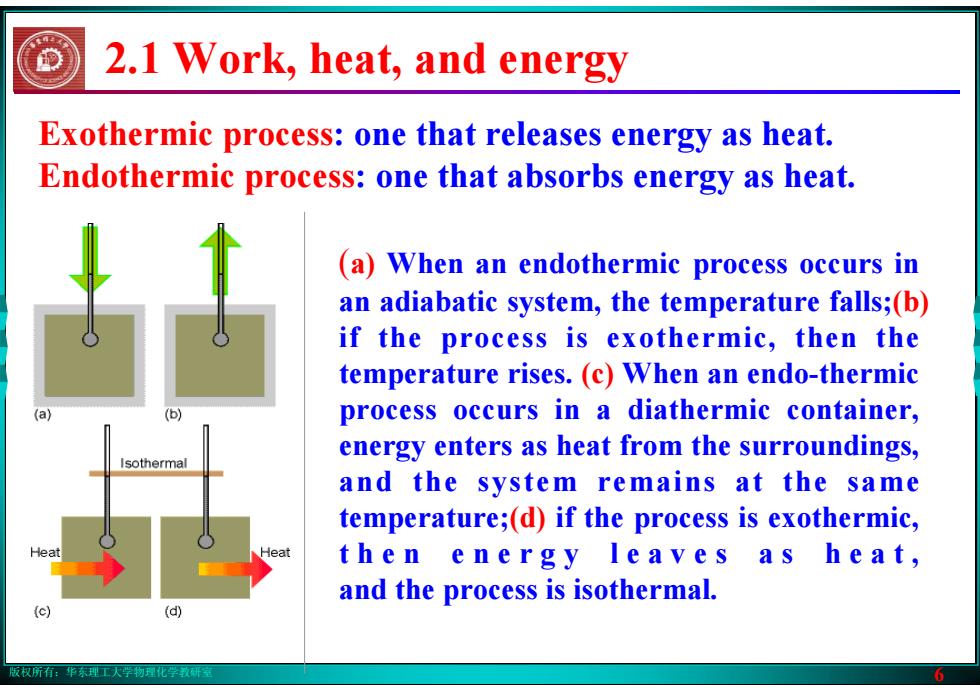
版权所有:华东理工大学物理化学教研室 6 Exothermic process: one that releases energy as heat. Endothermic process: one that absorbs energy as heat. (a) When an endothermic process occurs in an adiabatic system, the temperature falls;(b) if the process is exothermic, then the temperature rises. (c) When an endo-thermic process occurs in a diathermic container, energy enters as heat from the surroundings, and the system remains at the same temperature;(d) if the process is exothermic, t h e n e n e r g y l e a v e s a s h e a t , and the process is isothermal. 2.1 Work, heat, and energy
版权所有:华东理工大学物理化学教研室 6 Exothermic process: one that releases energy as heat. Endothermic process: one that absorbs energy as heat. (a) When an endothermic process occurs in an adiabatic system, the temperature falls;(b) if the process is exothermic, then the temperature rises. (c) When an endo-thermic process occurs in a diathermic container, energy enters as heat from the surroundings, and the system remains at the same temperature;(d) if the process is exothermic, t h e n e n e r g y l e a v e s a s h e a t , and the process is isothermal. 2.1 Work, heat, and energy
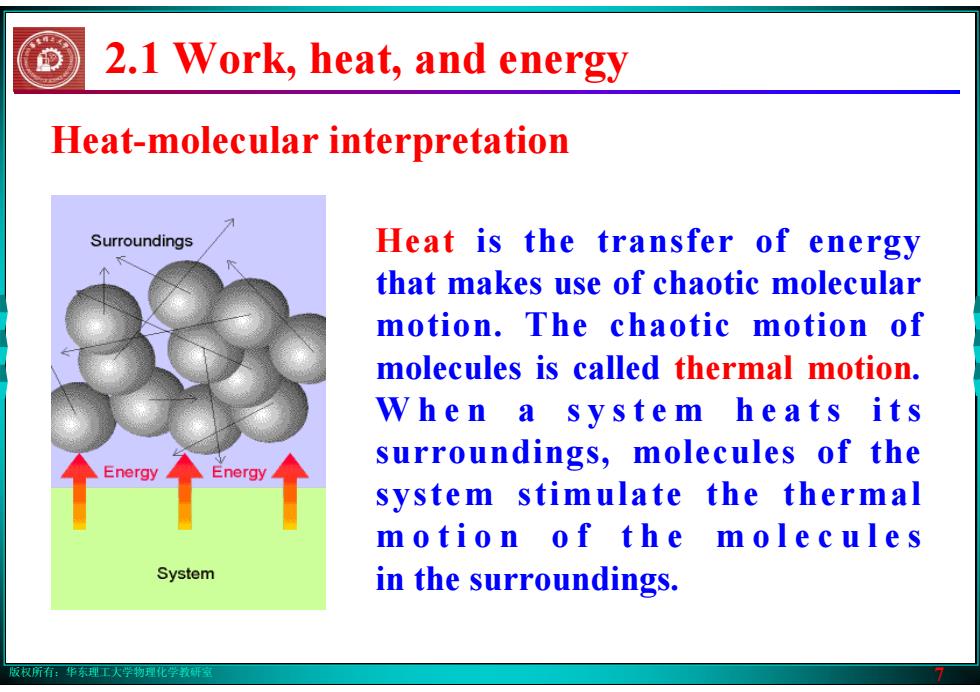
版权所有:华东理工大学物理化学教研室 7 Heat-molecular interpretation Heat is the transfer of energy that makes use of chaotic molecular motion. The chaotic motion of molecules is called thermal motion. W h e n a s y s t e m h e a t s i t s surroundings, molecules of the system stimulate the thermal m o t i o n o f t h e m o l e c u l e s in the surroundings. 2.1 Work, heat, and energy
版权所有:华东理工大学物理化学教研室 7 Heat-molecular interpretation Heat is the transfer of energy that makes use of chaotic molecular motion. The chaotic motion of molecules is called thermal motion. W h e n a s y s t e m h e a t s i t s surroundings, molecules of the system stimulate the thermal m o t i o n o f t h e m o l e c u l e s in the surroundings. 2.1 Work, heat, and energy
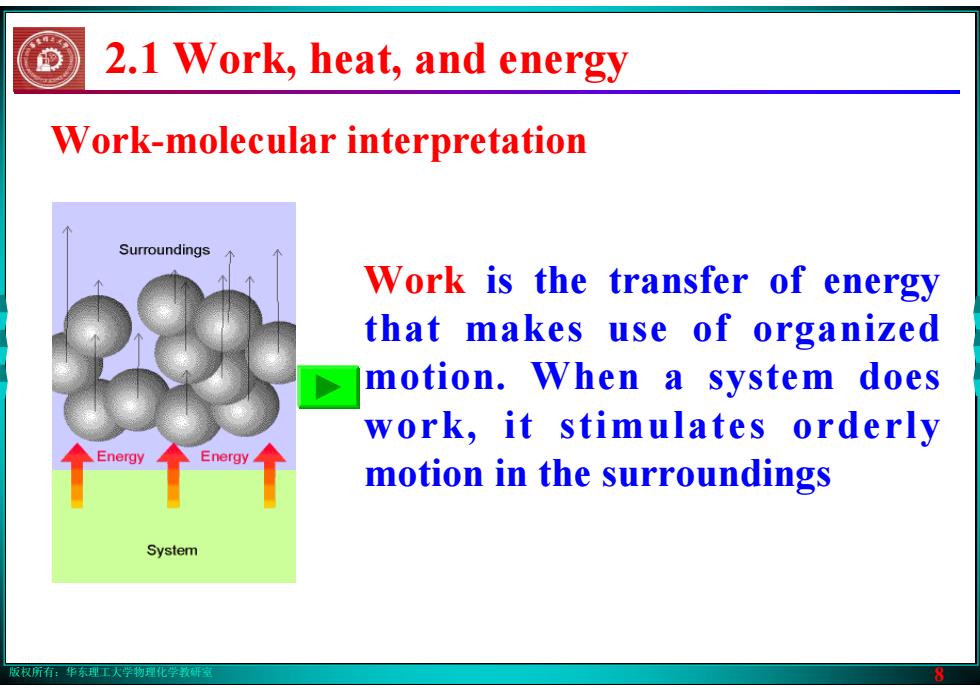
版权所有:华东理工大学物理化学教研室 8 Work-molecular interpretation Work is the transfer of energy that makes use of organized motion. When a system does work, it stimulates orderly motion in the surroundings 2.1 Work, heat, and energy
版权所有:华东理工大学物理化学教研室 8 Work-molecular interpretation Work is the transfer of energy that makes use of organized motion. When a system does work, it stimulates orderly motion in the surroundings 2.1 Work, heat, and energy

版权所有:华东理工大学物理化学教研室 9 The basic concepts 2.1 Work, heat, and energy 2.2 The First Law 1). The internal energy 2). The conservation of energy 3). The formal statement of the First Law
版权所有:华东理工大学物理化学教研室 9 The basic concepts 2.1 Work, heat, and energy 2.2 The First Law 1). The internal energy 2). The conservation of energy 3). The formal statement of the First Law
版权所有:华东理工大学物理化学教研室 10 The internal energy, U : the total energy of a system is called its internal energy;it is the total kinetic and potential energy of the molecules composing the system. The internal energy is a state function. It is a extensive property. Internal energy, heat, and work are measured in the same units, the Joule (J). 2.2 The First Law 1). The internal energy
版权所有:华东理工大学物理化学教研室 10 The internal energy, U : the total energy of a system is called its internal energy;it is the total kinetic and potential energy of the molecules composing the system. The internal energy is a state function. It is a extensive property. Internal energy, heat, and work are measured in the same units, the Joule (J). 2.2 The First Law 1). The internal energy