
·(3)化学计量点 ·溶液中c(C)来源于AgC的离解,此时溶 液的c(C)、c(Ag)相等,即: ● c(CI)=c(Ag)=VKm.g =/1.8×1010=1.34x105mol/L pC1=-lgc(C)=4.87
• (3) 化学计量点 • 溶液中c(Cl- )来源于AgCl↓的离解,此时溶 液的c(Cl- )、c(Ag+)相等,即: • • pCl = - lgc(Cl- ) = 4.87 , 10 5 ( ) ( ) 1.8 10 1.34 10 / sp AgCl c Cl c Ag K mol L − + − − = = = =

(4)计量点后 ·溶液中c(C)决定于过量AgNO3的量,当滴入 AgNO3溶液20.02mL时 (Ag)=V(AgNO)-V(NaCDk(AgNO,) V(NaCl)+V(AgNO:) c(C)=Kpdga/c(Ag) c(Ag)=5.00×105mol/L pC1=pKm-pAg=9.74-4.30=5.44
• (4) 计量点后 • 溶液中c(Cl- )决定于过量AgNO3的量, 当滴入 AgNO3溶液20.02mL时 ( ) ( ) ( ) ( ) ( ) ( ) 3 3 3 V NaCl V AgNO V AgNO V NaCl c AgNO c Ag + − = + ( ) ( ) , − + c Cl = Ksp AgCl c Ag 5 ( ) 5.00 10 / 9.74 4.30 5.44 sp c Ag mol L pCl pK pAg + − = = − = − =
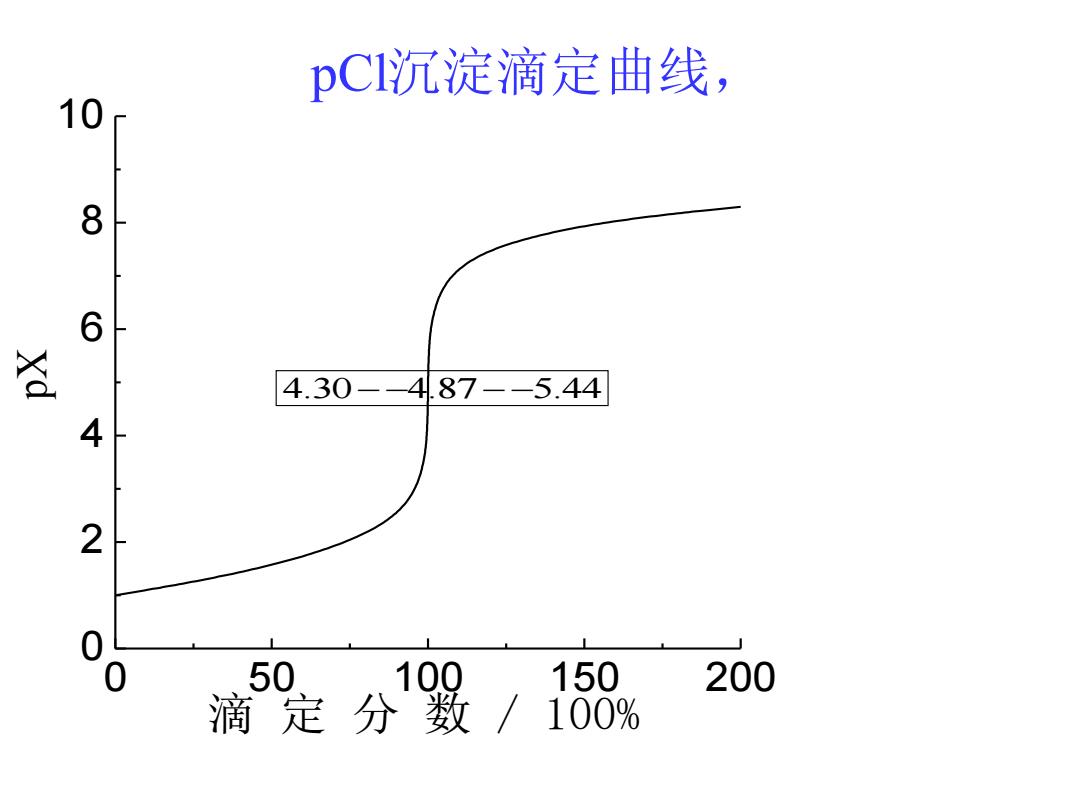
pCI沉淀滴定曲线, 10 8 6 签 4.30--487--5.44 4 2 0 50 100 150 200 滴定分数 100%
pCl沉淀滴定曲线, 4.30 4.87 5.44 − − − − 0 50 100 150 200 0 2 4 6 8 10 pX 滴 定 分 数 / 100%