
可逆与不可逆讨论(例1) 可逆热机 300 2000K 7=1- 0.85 2000 100kJ W =7,9=0.85×100=85kJ 85 kJ ASiso =AST +AScycle +AST: 15 kJ -100 15 +0+ =0 2000 300 300K
可逆与不可逆讨论 ( 例1) 可逆热机 2000 K 300 K 100 kJ 15 kJ 85 kJ t 300 1 0.85 2000 η =− = 1 2 iso T cycle T 100 15 0 0 2000 300 Δ =Δ +Δ +Δ S SS S − = ++ = W Q = ηt 1 = ×= 0.85 100 85kJ
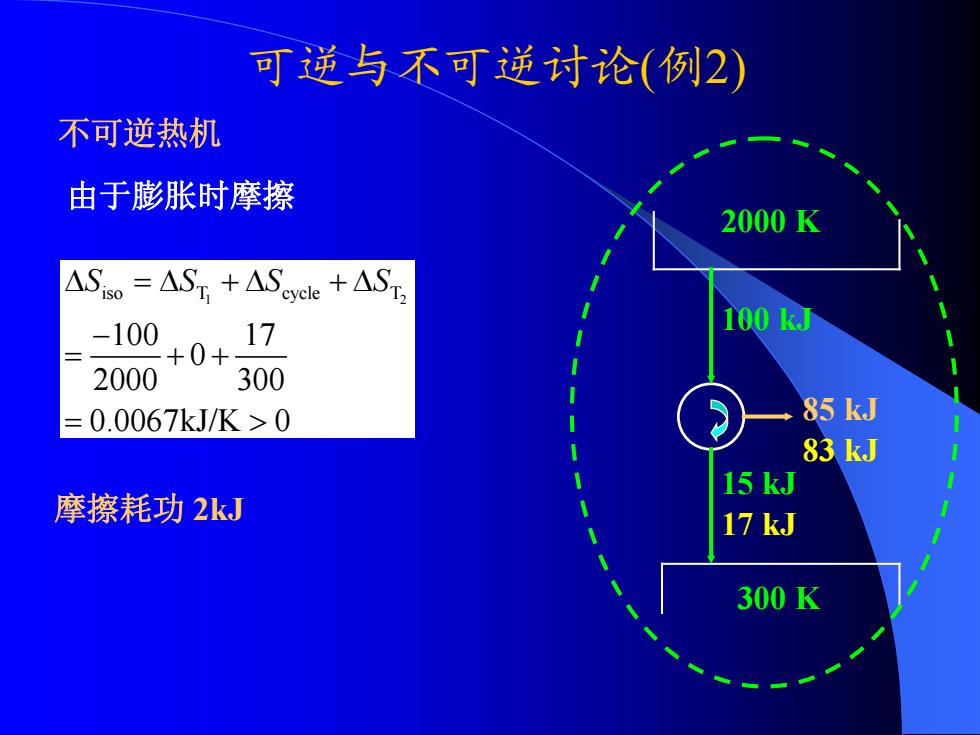
可逆与不可逆讨论(例2) 不可逆热机 由于膨胀时摩擦 2000K ASio =AST +AScycke +AST, -100 17 100k周 +0+ 2000 300 =0.0067kJ/K>0 85 kJ 83 kJ 15 kJ 摩擦耗功2kJ 17 kJ 300K
可逆与不可逆讨论 ( 例2) 2000 K 300 K 100 kJ 15 kJ 85 kJ 1 2 iso T cycle T 100 17 0 2000 300 0.0067kJ/K 0 Δ =Δ +Δ +Δ S SS S − = ++ = > 不可逆热机 83 kJ 17 kJ 由于膨胀时摩擦 摩擦耗功 2kJ
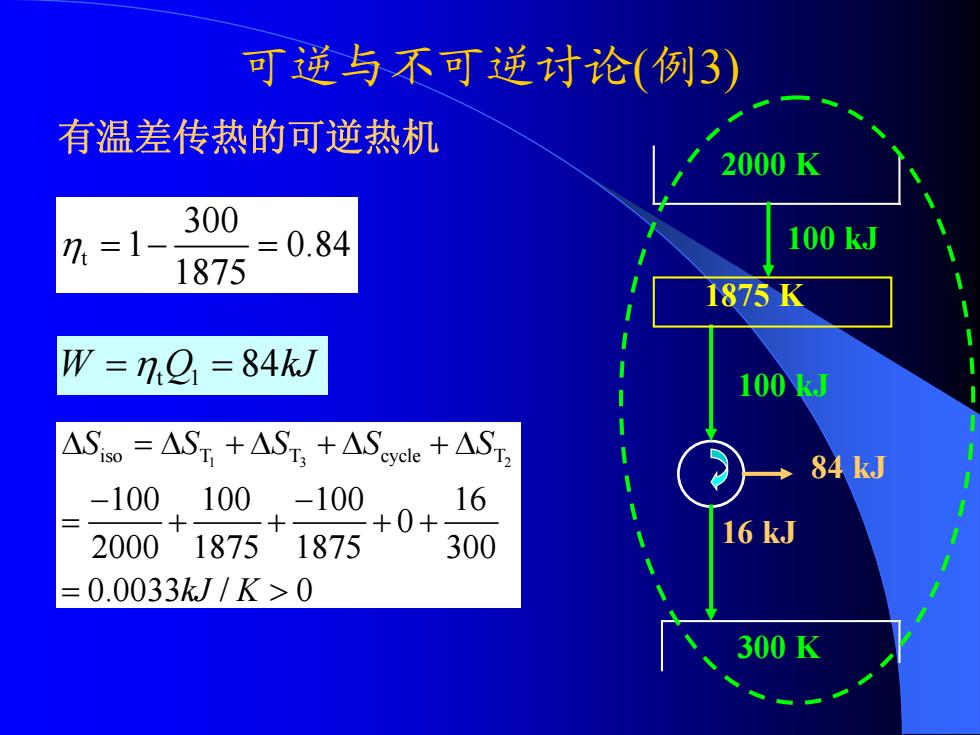
可逆与不可逆讨论(例3) 有温差传热的可逆热机 2000K 300 7=1 0.84 100kJ 1875 1875K W =7.9=84k/ 100J ASiso=AST +AST,+ASeycle +AST 84 kJ -100100-100 。16 +0+ 200018751875 300 16 kJ =0.0033kJ/K>0 300K
可逆与不可逆讨论 ( 例3) 有温差传热的可逆热机 2000 K 300 K 100 kJ 16 kJ 84 kJ t 300 1 0.84 1875 η =− = 1 3 2 iso T T cycle T 100 100 100 16 0 2000 1875 1875 300 0.0033 / 0 S SSS S kJ K Δ =Δ +Δ +Δ +Δ − − = + + ++ = > W Q kJ = ηt 1 = 84 100 kJ 1875 K
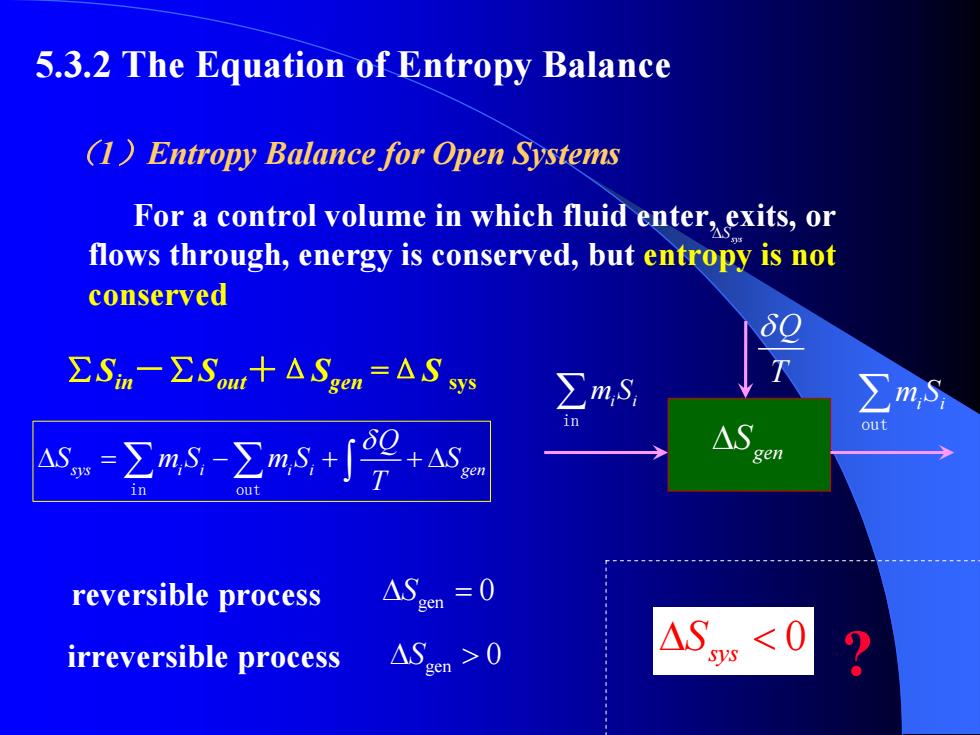
5.3.2 The Equation of Entropy Balance (1)Entropy Balance for Open Systems For a control volume in which fluid enter,exits,or flows through,energy is conserved,but entropy is not conserved ∑Sn-∑Som+△Sen=△Ssy ∑m,s i A-∑m-∑ms+∫9 △S 011 +△S en en out reversible process △Sm=0 irreversible process ASn≥0
5.3.2 The Equation of Entropy Balance sys i i i i gen Q S mS mS S T δ Δ = − + +Δ ∑ ∑ ∫ in out sys Δ S (1 )Entropy Balance for Open Systems For a control volume in which fluid enter, exits, or flows through, energy is conserved, but entropy is not conserved ∑ Sin-∑ Sout+Δ Sgen = Δ S sys ∑m Si i in ∑m Si i out Q T δ gen Δ S gen reversible process Δ S = 0 irreversible process gen Δ S > 0 0 sys Δ S < ?
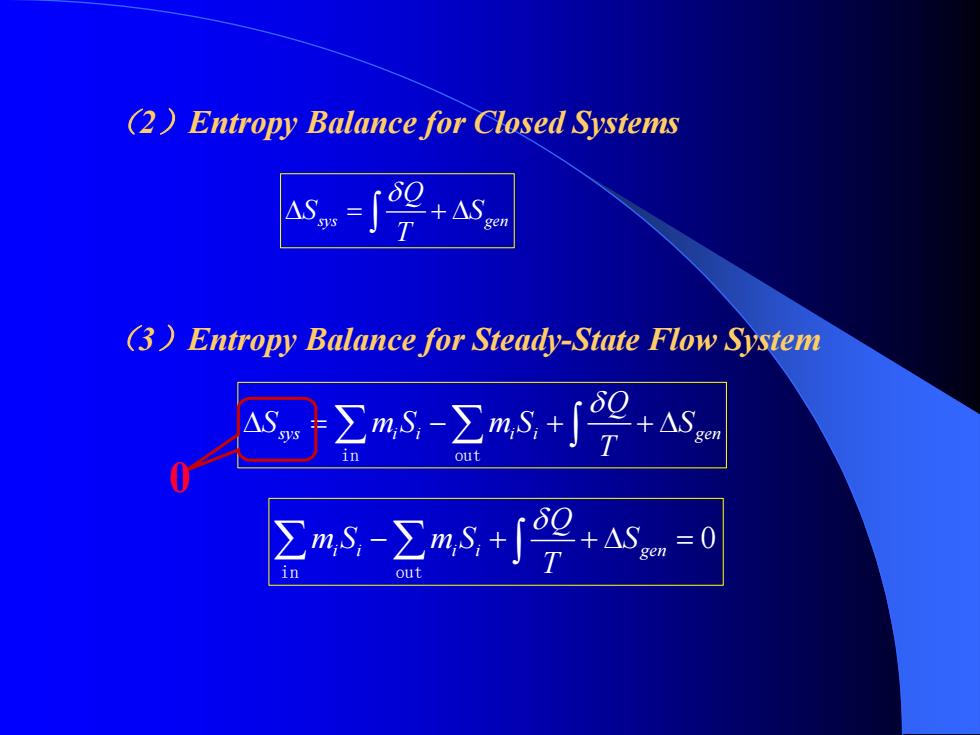
(2)Entropy Balance for Closed Systems T (3)Entropy Balance for Steady-State Flow System ∑mN-∑ms+9心 ∑mS-∑ms+∫2+心=0 i
sys gen Q S S T δ Δ = +Δ ∫ sys i i i i gen Q S mS mS S T δ Δ = − + +Δ ∑ ∑ ∫ in out (2)Entropy Balance for Closed Systems (3)Entropy Balance for Steady-State Flow System 0 0 ii ii gen Q mS mS S Tδ ∑ ∑− + +Δ = ∫ in out