
over the CHAPTER 17 Aldehydes and Ketones The Carbonyl Group Thenpaced be cm 8aagr2号nosc wagotn9teoalnone,6niebnvaeSycem 岩小 9990 theending of the allane s NecSpmomegae8ceP2R5egctennmpkstketone,popanom a-d 云管aaw,m names 1
1 CHAPTER 17 Aldehydes and Ketones: The Carbonyl Group 17-1 Naming the Aldehydes and Ketones The aldehyde function takes precedence over the ketone group in nomenclature. Simpler aldehydes often retain their common names. These are constructed by dropping the –ic or –oic acid ending and replacing it with –aldehyde: Many ketones also have common names consisting of the substituent names followed by the word ketone. Dimethyl ketone is best known as acetone. Phenyl ketones have common names ending in –phenone. IUPAC nomenclature treats aldehydes as derivatives of alkanes. The ending –e is replaced by –al. An alkane becomes an alkanal. Chem Abstracts retains the common names of the first two aldehydes: The names parallel those of the 1-alkanols except that the aldehyde carbon is assumed to be C1 and does not have to be specified. When an aldehyde is attached to a ring it is called a carbaldehyde. The carbon atom bearing the aldehyde is C1. The simplest aromatic aldehyde, benzenecarbaldehyde, is referred to using its common name, benzaldehyde, by Chem Abstracts. Ketones are called alkanones, the ending –e of the alkane is replaced with –one. IUPAC provides an exception for the simplest ketone, propanone, the common name of which is acetone. The carbonyl carbon of a ketone is assigned the smallest possible number, regardless of the presence of other substituents or the – OH, C=C or C≡C functional groups. Aromatic ketones are named as aryl-substituted alkanones. Ketones are called cycloalkanones when part of a ring. The carbonyl carbon is assigned C1 in this case. The systematic name of the fragment is “alkanoy.” The older term “acyl” is widely used. Both IUPAC and Chemical Abstracts retain the older names “formyl” and “acetyl” for the groups and . The term “oxo” denotes the location of a ketone carbonyl group when present with an aldehyde function

17-2 Structure of the Carbonyl Group toteeupcontaihnsasho rt,strong and y0aaiom9 ow up入又, 9≥9c8 he&edmsarterratgenot他enaeannopobitalon o 影 One of the simplest aldehyde is acetaldehyde .The oxygen contains two lone pairs in two sp?orbitals o6 H.e Deseriptiens of a Carbenyl Group 1-rieoyde ad IeorR,aesheaed.heeamgat6nomanaaenvoes his is caused by two fact lypoitvecarbonyncaus ibie with wster.As the 2
2 Several conventions can be used to denote the structure of an aldehyde or ketone: The condensed formula of an aldehyde must be written as RCHO and not RCOH, which might be confused with the hydroxy group of an alcohol. 17-2 Structure of the Carbonyl Group The carbonyl group contains a short, strong and very polar bond. The hybridization of the C and O atoms of the carbonyl group are sp2 hybridized. The C and O atoms of the carbonyl group and the two atoms attached to the carbon all lie in the same plane. The bond angles about the carbonyl are about 120o. The π bond of the carbonyl is made of the remaining p orbital on the carbon and a similar p orbital on the oxygen. One of the simplest aldehyde is acetaldehyde: The primary electronic differences between a carbonyl group and an ordinary double bond are: •The oxygen contains two lone pairs in two sp2 orbitals; •The oxygen is more electronegative than carbon. The C=O bond is polarized; the carbon possesses a slight positive charge and the oxygen a slight negative charge. Polarization alters the physical constants of aldehydes and ketones. Due to the polarization of their carbonyl groups, the boiling points of aldehydes and ketones are higher than hydrocarbons of similar molecular weights. Acetaldehyde and acetone are completely miscible with water. As the hydrocarbon length increases, solubility decreases. Carbonyl compounds have more than six carbons and are relatively insoluble in water. Spectroscopic Properties of Aldehydes and Ketones 17-3 The 1H NMR spectrum of the formyl hydrogen of an aldehyde is very strongly deshielded, appearing at 9-10 ppm. This is caused by two factors: •Similar to alkenes, the movement of the π electrons in the magnetic field strengthens the external field; •The partially positive carbonyl carbon causes additional deshielding
eand ketones sho rbonyl carbon deshielded compared to slightly deshielded by the partially meaeSepeiacarbongroupsdirecyndicatednan :ntesban-0m Acydic alka one:about 1715 cm 的 9% 3
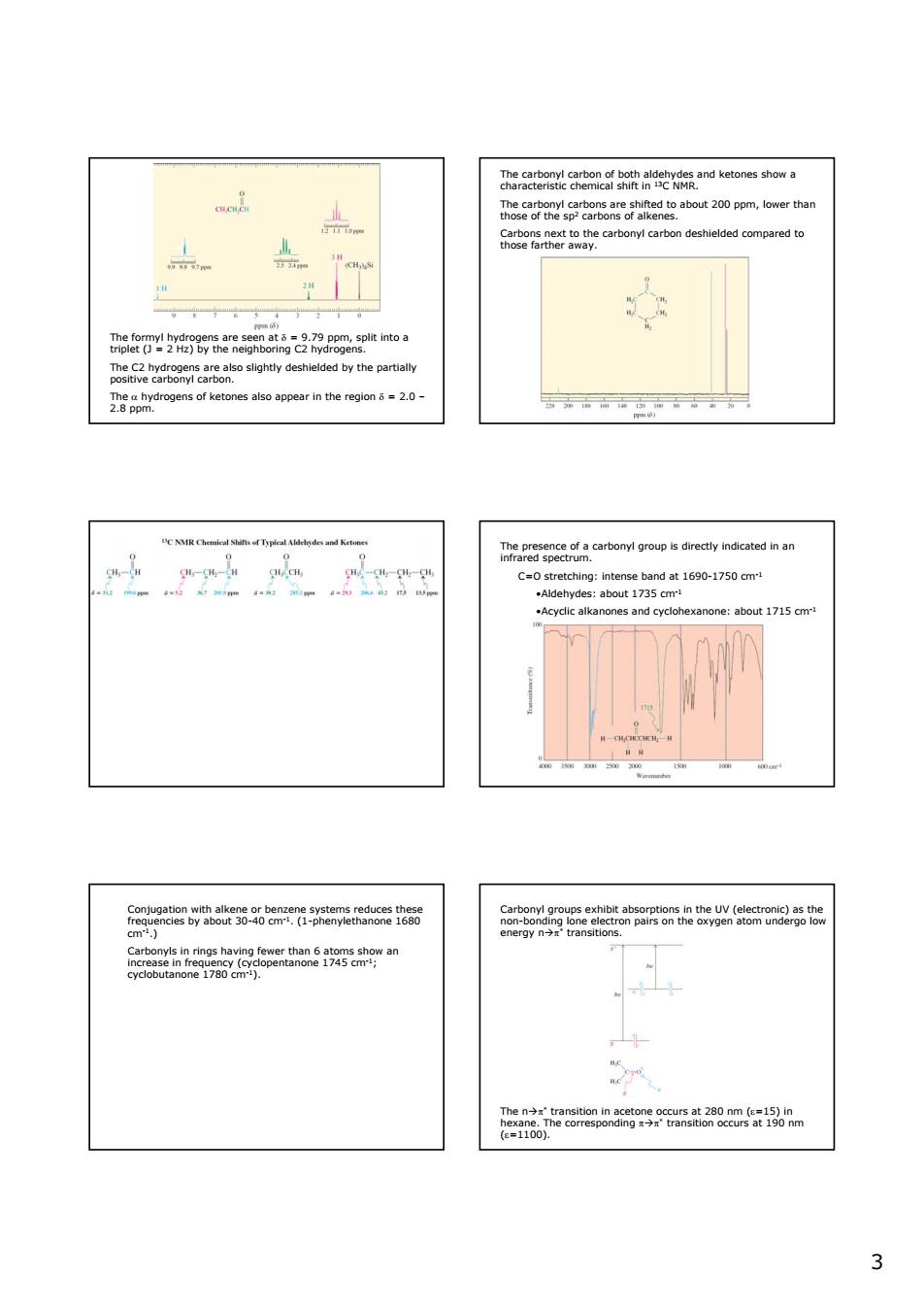
3 The formyl hydrogens are seen at δ = 9.79 ppm, split into a triplet (J = 2 Hz) by the neighboring C2 hydrogens. The C2 hydrogens are also slightly deshielded by the partially positive carbonyl carbon. The α hydrogens of ketones also appear in the region δ = 2.0 – 2.8 ppm. The carbonyl carbon of both aldehydes and ketones show a characteristic chemical shift in 13C NMR. The carbonyl carbons are shifted to about 200 ppm, lower than those of the sp2 carbons of alkenes. Carbons next to the carbonyl carbon deshielded compared to those farther away. The presence of a carbonyl group is directly indicated in an infrared spectrum. C=O stretching: intense band at 1690-1750 cm-1 •Aldehydes: about 1735 cm-1 •Acyclic alkanones and cyclohexanone: about 1715 cm-1 Conjugation with alkene or benzene systems reduces these frequencies by about 30-40 cm-1. (1-phenylethanone 1680 cm-1.) Carbonyls in rings having fewer than 6 atoms show an increase in frequency (cyclopentanone 1745 cm-1; cyclobutanone 1780 cm-1). Carbonyl groups exhibit absorptions in the UV (electronic) as the non-bonding lone electron pairs on the oxygen atom undergo low energy nÆπ* transitions. The nÆπ* transition in acetone occurs at 280 nm (ε=15) in hexane. The corresponding πÆπ* transition occurs at 190 nm (ε=1100)

ggonoa2egiahdaonsshstne keoneepoeleemeaiatanaealnetodesand Elctrnic Tranitions fAcetone and 3-Buten--one 网gm6g3G CH.CCH CH-CHecH, 调 M-CHy The symmetric ketone generates a single acylium peak 4
4 Conjugation of carbonyl groups with double bonds shifts the absorption to longer wavelengths: Mass spectral fragmentation of aldehydes and ketones provides structural information. 2-Pentanone, 3-pentanone and 3-methyl-2-butanone all fragment by α cleavage yielding the corresponding acylium cation and an alkyl radical. Note that the two acylium fragments identify the composition of the ketone. The peak at mass 58 results from a McLafferty rearrangement. This occurs in compounds having a hydrogen atom γ to the carbonyl oxygen and enough flexibility to allow them to get close together. This extra peak allows 2-pentanone to be distinguished from 3-methyl-2- butanone, which give otherwise identical fragments. The symmetric ketone generates a single acylium peak. Note the absence of a peak at 58, which is present in the mass spectrum of 2-pentanone

17-Preparation of Aldehydes and Ketones Oxidation of alcohols: bboronmheiaofaldahydesandketonesus As-e5络时e 四。 Ozonol小sis rleuaone 85 17-Reactivity of the Carbonyl Group:Mechanisms of Friedel-Crufts Alkanoylation (Acylation) Regons f Reactiity Aldchydesand Ketemes 5
5 17-4 Preparation of Aldehydes and Ketones Laboratory syntheses of aldehydes and ketones use four common methods. Oxidation of alcohols: Ozonolysis: Hydration of the carbon-carbon triple bond yields enols that tautomerize to carbonyl compounds. In the presence of mercuric ion, addition of water follows Markovnikov’s rule to furnish ketones. Actual electronic control. Friedel-Crafts alkanoylation (acylation): Reactivity of the Carbonyl Group: Mechanisms of Addition 17-5 There are three regions of reactivity in aldehydes and ketones