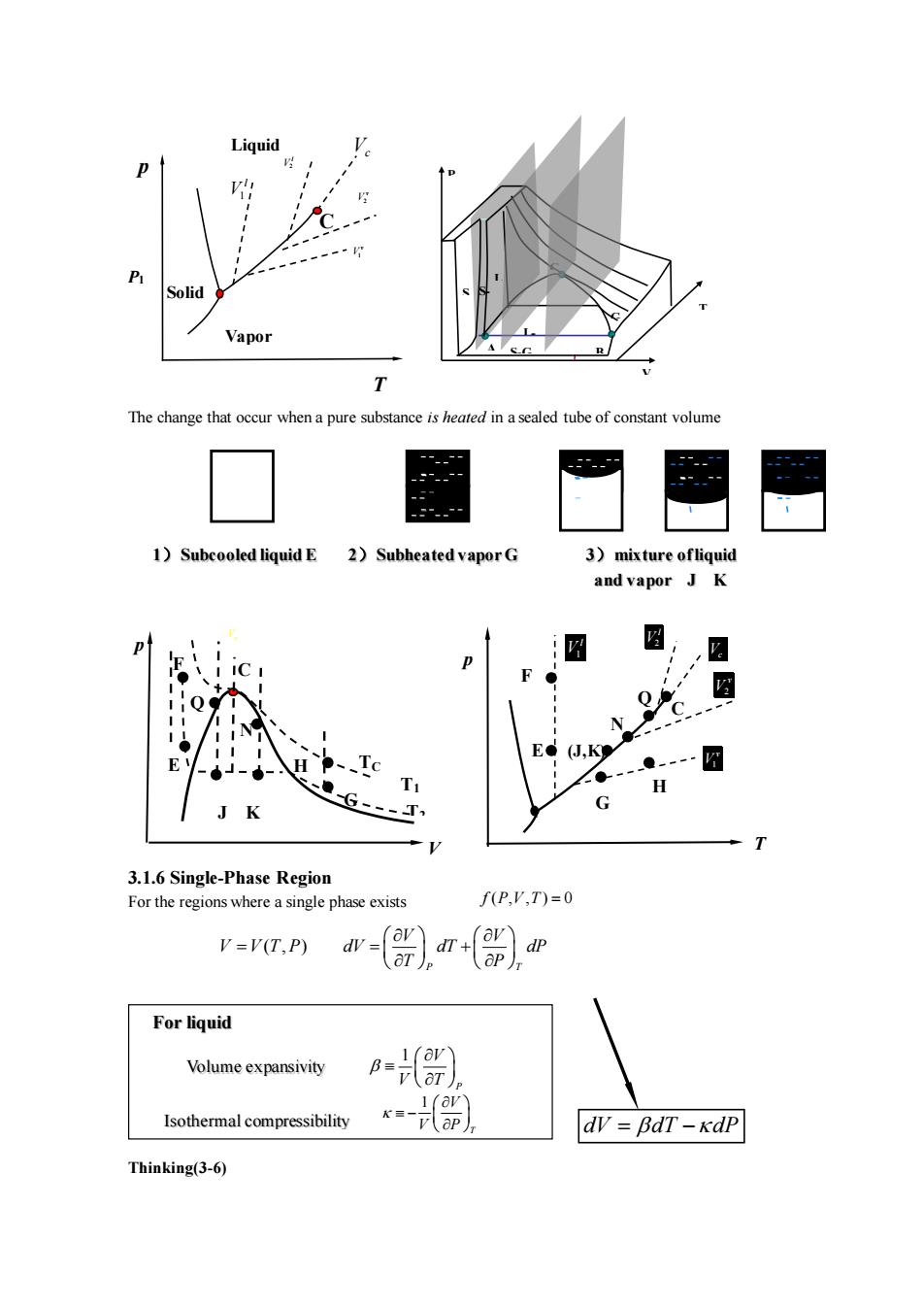
Liauid Vapor The change that occur whena pure substanceis heed nasealed tube of constant volume - 1)Subcooled liquidE 2)Subheated vaporG 3)mixture ofliquid and vapor J K pt 图 ● Q N (,K ●- H G-T T 3.1.6 Single-Phase Region For the regions where a single phase exists f(P,',T)=0 V=V(T.P) w-r)n) Forliquid Volume expansivity =】 Isothermal compressibility dv BdT-kdP Thinking(3-6)
The change that occur when a pure substance is heated in a sealed tube of constant volume 3.1.6 Single-Phase Region For the regions where a single phase exists Thinking(3-6) p T C V T1 TC C T2 p F E E F (J,K) H G N Q Vc 2 v V 1 v V 2 l V 1 l V J K N Q G H Vc V V T P = ( , ) P T V V dV dT dP T P = + Isothermal compressibility 1 P V V T 1 T V V P − Volume expansivity dV dT dP = − For liquid V L P T A B C S G L- S-GG S- L p T Liquid Vapor Solid C P1 1 l V 2 l V Vc 1 v V 2 v V 1)Subcooled liquid E 2)Subheated vapor G 3)mixture ofliquid and vapor J K f P V T ( , , ) 0 =
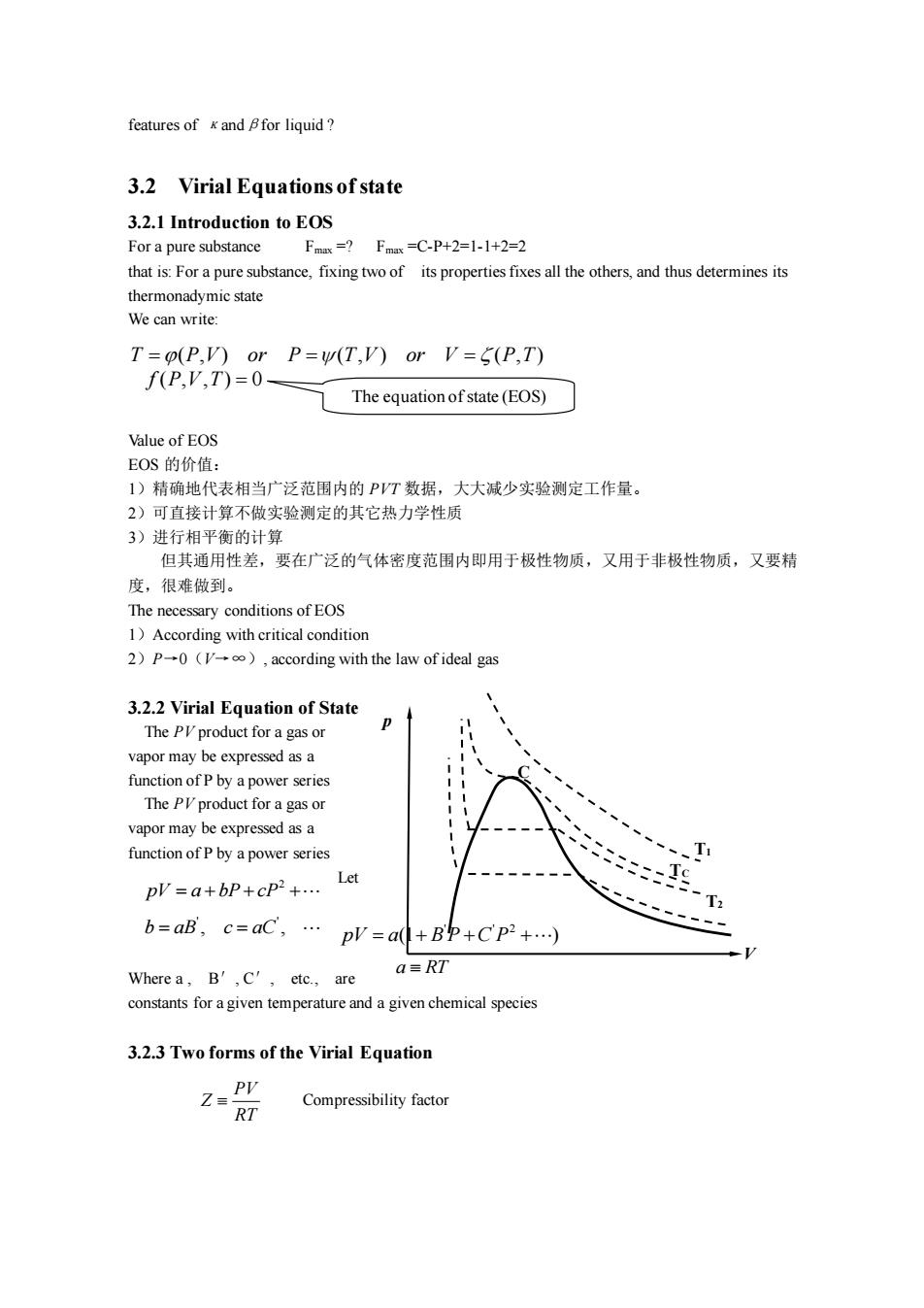
features of and for liquid? 3.2 Virial Equations of state 3.2.I Introduction to EOS For a pure substance F=1Fx=C-P+2=1-1+2=2 that is:For a pure substance.fixing two of its properties fixes all the othersand thus determines its T=o(P,V)or P=W(T,V)or V=5(P,T) f(P.V,T)=0- The equation ofstate(EOS) Value of EOS EOS的价值: 1)精确地代表相当广泛范围内的PT数据,大大减少实验测定工作量。 2)可直接计算不做实验测定的其它热力学性质 3)讲行相平衡的计算 但其通用性差,要在广泛的气体密度范围内即用于极性物质,又用于非极性物质,又要精 度,很难做到。 The necessary conditions of EOS 1)According with critical condition 2)P0().according with the law of ideal gas 3.2.2 Virial Equation of State The PIproduct for a gas or vapor may be expressed as a function of P by a power series s or The PI product for a gas vapor may be expressed as a function of Pby a power series plV=a+bP+cP2+. Let c9 T b=aB,c=aC,.p=a+Bp+Cp2+) Wherea.B',C',etc.,are a=RT constants foragiven temperature and a given chemical species 3.2.3 Two forms of the Virial Equation Compressibility factor RT
features of κandβfor liquid ? 3.2 Virial Equations of state 3.2.1 Introduction to EOS For a pure substance Fmax =? Fmax =C-P+2=1-1+2=2 that is: For a pure substance, fixing two of its properties fixes all the others, and thus determines its thermonadymic state We can write: Value of EOS EOS 的价值: 1)精确地代表相当广泛范围内的 PVT 数据,大大减少实验测定工作量。 2)可直接计算不做实验测定的其它热力学性质 3)进行相平衡的计算 但其通用性差,要在广泛的气体密度范围内即用于极性物质,又用于非极性物质,又要精 度,很难做到。 The necessary conditions of EOS 1)According with critical condition 2)P→0(V→∞), according with the law of ideal gas 3.2.2 Virial Equation of State The PV product for a gas or vapor may be expressed as a function of P by a power series The PV product for a gas or vapor may be expressed as a function of P by a power series Let Where a , B′, C′, etc., are constants for a given temperature and a given chemical species 3.2.3 Two forms of the Virial Equation Compressibility factor T P V or P T V or V P T = = = ( , ) ( , ) ( , ) The equation of state (EOS) f P V T ( , , ) 0 = T1 p TC C T2 V 2 pV a bP cP = + + + ' ' b aB c aC = = , , ' ' 2 pV a B P C P = + + + (1 ) a RT PV Z RT