3.1 PIT behavior of pure substances By measuring the vapor pressure,volume and temperature of a pure substances when different phases achieve equilibrium,we can draw the curve of P-V-T V=constant PT diagram T=constant PV diagram P=constant TV diagram PVT PTV diagram
3.1 PVT behavior of pure substances By measuring the vapor pressure, volume and By measuring the vapor pressure, volume and temperature of a pure substances when different temperature of a pure substances when different phases achieve equilibrium, we can draw the curve of phases achieve equilibrium, we can draw the curve of P-V-T V=constant PT diagram T=constant PV diagram P=constant TV diagram P V T PTV diagram
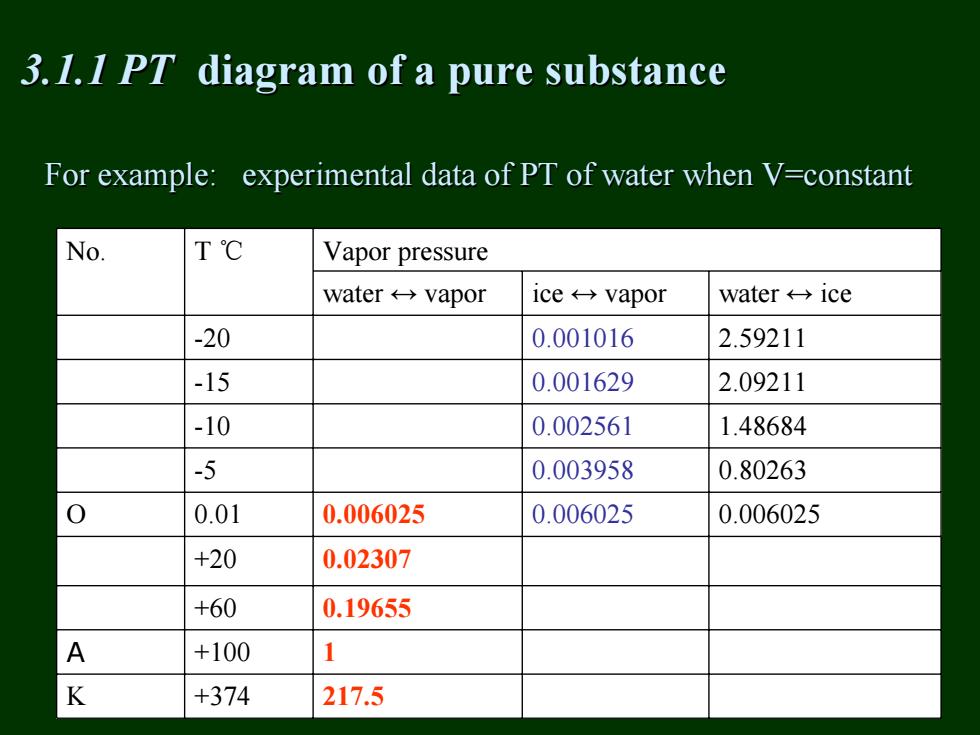
3.1.I PT diagram of a pure substance For example:experimental data of PT of water when V=constant No. T℃ Vapor pressure water←→vapor ice←→vapor water←→ice -20 0.001016 2.59211 -15 0.001629 2.09211 -10 0.002561 1.48684 -5 0.003958 0.80263 0.01 0.006025 0.006025 0.006025 +20 0.02307 +60 0.19655 A +100 1 K +374 217.5
For example: experimental data of PT of water when V=constant For example: experimental data of PT of water when V=constant No. T ℃ Vapor pressure water ↔ vapor ice ↔ vapor water ↔ ice -20 0.001016 2.59211 -15 0.001629 2.09211 -10 0.002561 1.48684 -5 0.003958 0.80263 O 0.01 0.006025 0.006025 0.006025 +20 0.02307 +60 0.19655 A +100 1 K +374 217.5 3.1.1 PT 3.1.1 PT diagram diagram of a pure substance of a pure substance
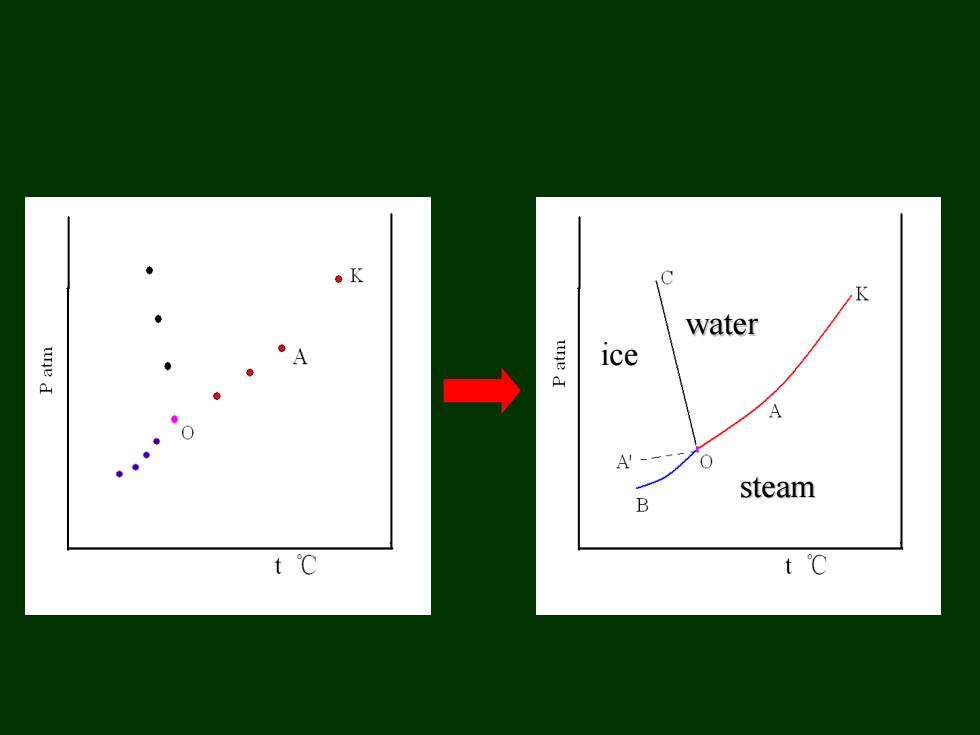
。K C K water une d A ice → 。 A'- 0 steam B t℃ t℃
water steam ice
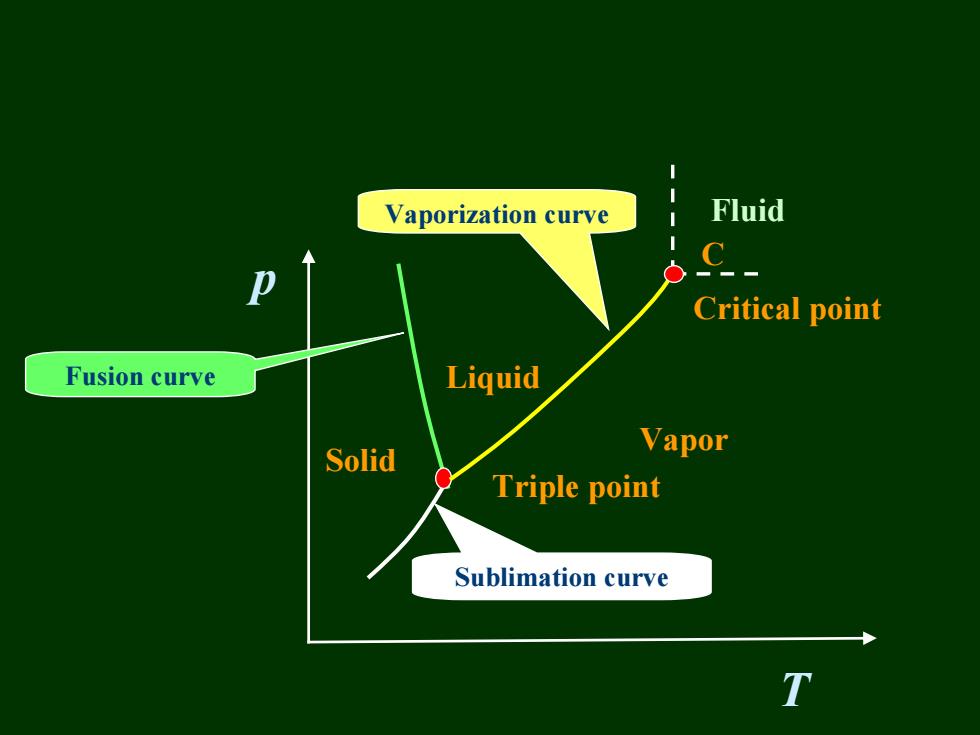
Vaporization curve Fluid Critical point Fusion curve Liquid Solid Vapor Triple point Sublimation curve
p T Liquid Vapor Solid Triple point Critical point Sublimation curve Fusion curve Vaporization curve Fluid C
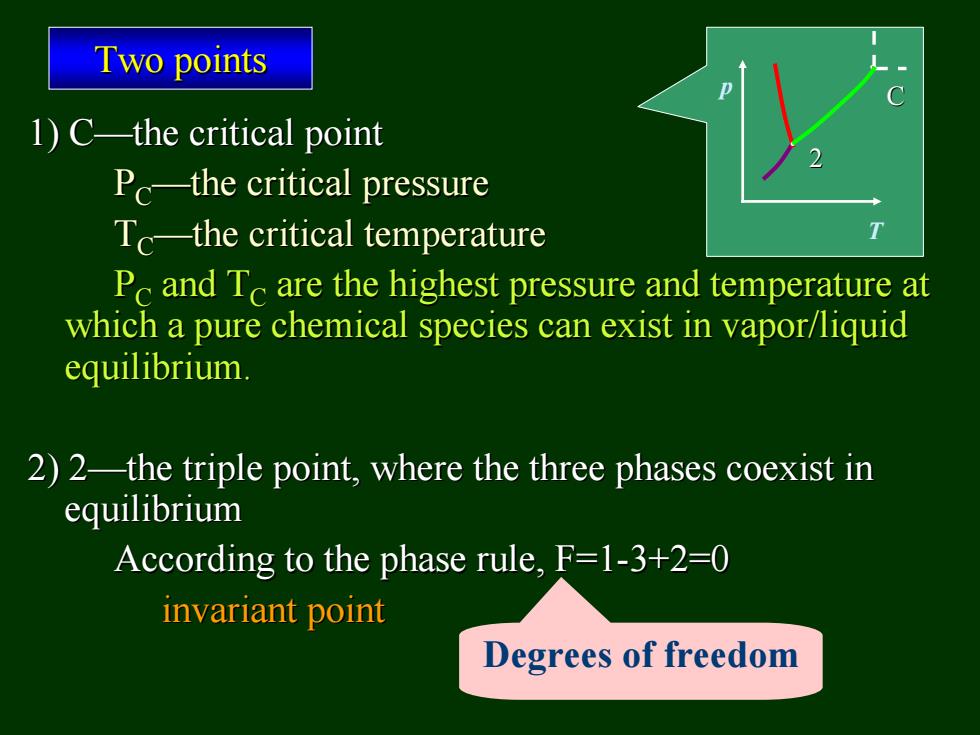
Two points 1)C-the critical point Pc-the critical pressure Tc-the critical temperature Pc and Tc are the highest pressure and temperature at which a pure chemical species can exist in vapor/liquid equilibrium. 2)2-the triple point,where the three phases coexist in equilibrium According to the phase rule,F=1-3+2=0 invariant point Degrees of freedom
1) C—the critical point the critical point PC—the critical pressure the critical pressure TC—the critical temperature the critical temperature PC and TC are the highest pressure and temperature at are the highest pressure and temperature at which a pure chemical species which a pure chemical species can exist in vapor/liquid can exist in vapor/liquid equilibrium. equilibrium. 2) 2—the triple point, where the three phases coexist in the triple point, where the three phases coexist in equilibrium equilibrium According to the phase rule, F=1 According to the phase rule, F=1-3+2=0 3+2=0 invariant point invariant point Two points Two points p T 2 C Degrees of freedom