版权所有:华东理工大学物理化学教研室 25 1). Pressure, p Definition: Pressure is defined as force divided by the area to which the force is applied. Unit: Pascal (Pa), 1 Pa= 1 Nm-2; the SI unit. It is defined as 1 newton per meter squared; Standard pressure: , op 10 Pa 5 = o p 1.1 The states of gases The general form of an equation of state is: p= f (T,V,n)
版权所有:华东理工大学物理化学教研室 25 1). Pressure, p Definition: Pressure is defined as force divided by the area to which the force is applied. Unit: Pascal (Pa), 1 Pa= 1 Nm-2; the SI unit. It is defined as 1 newton per meter squared; Standard pressure: , op 10 Pa 5 = o p 1.1 The states of gases The general form of an equation of state is: p= f (T,V,n)
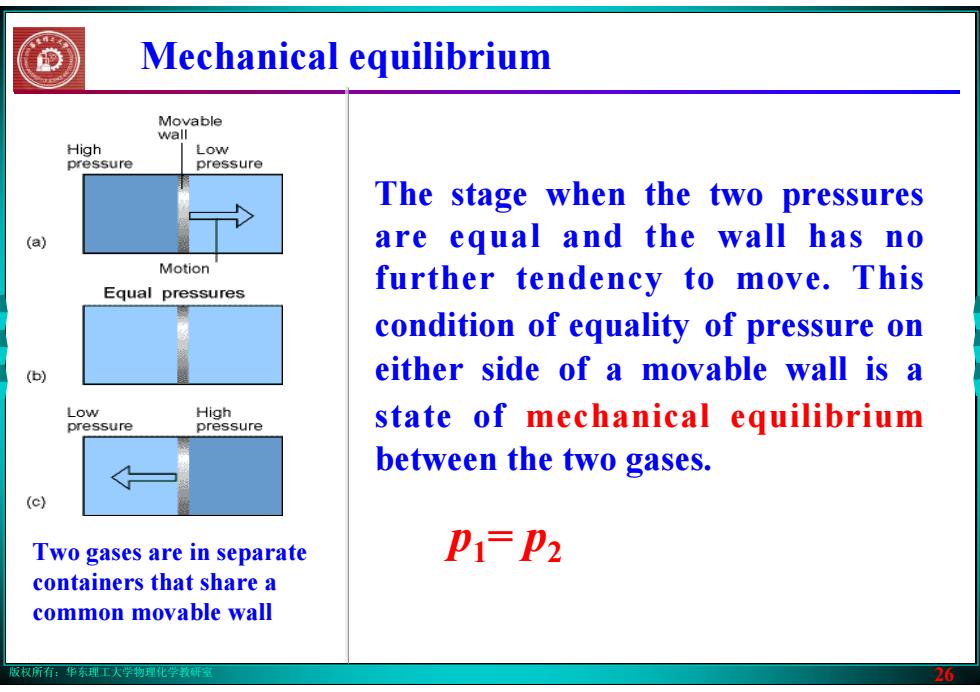
版权所有:华东理工大学物理化学教研室 26 Mechanical equilibrium Two gases are in separate containers that share a common movable wall The stage when the two pressures are equal and the wall has no further tendency to move. This condition of equality of pressure on either side of a movable wall is a state of mechanical equilibrium between the two gases. p1= p2
版权所有:华东理工大学物理化学教研室 26 Mechanical equilibrium Two gases are in separate containers that share a common movable wall The stage when the two pressures are equal and the wall has no further tendency to move. This condition of equality of pressure on either side of a movable wall is a state of mechanical equilibrium between the two gases. p1= p2
版权所有:华东理工大学物理化学教研室 27 2). Temperature, T Temperature scales: • The Celsius scale of temperature, θ, ℃ • The thermodynamic temperature scale, T, K T / K= θ, / ℃+273.15 The temperature is the property that tells us the direction of the flow of energy, 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 27 2). Temperature, T Temperature scales: • The Celsius scale of temperature, θ, ℃ • The thermodynamic temperature scale, T, K T / K= θ, / ℃+273.15 The temperature is the property that tells us the direction of the flow of energy, 1.1 The states of gases
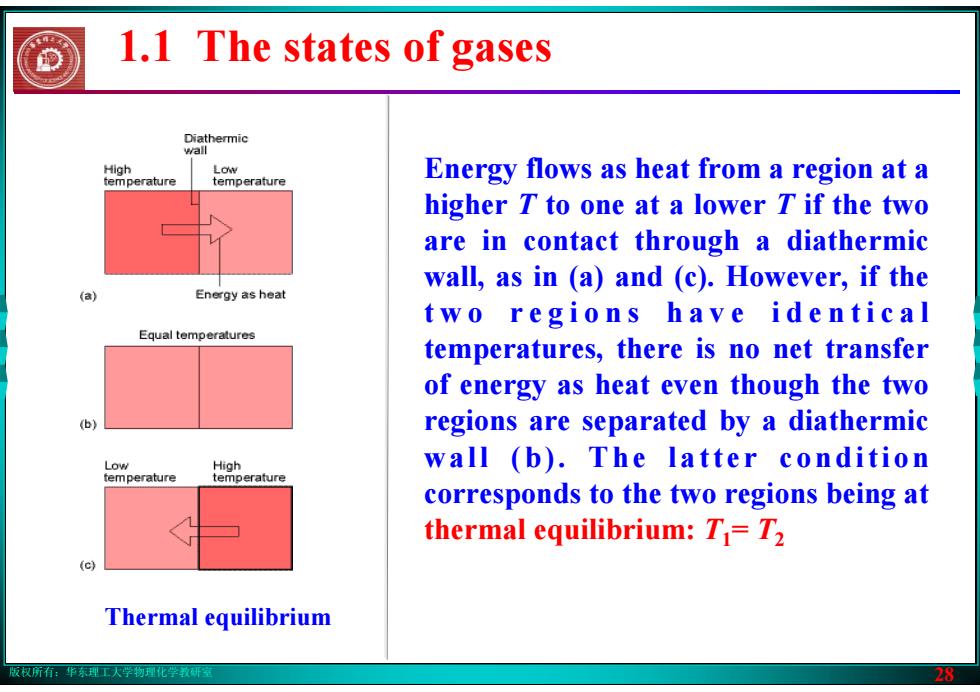
版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases

版权所有:华东理工大学物理化学教研室 29 The Zeroth Law of thermodynamics Zeroth Law of thermodynamics If an object A is in thermal equilibrium with B and if B is in thermal equilibrium with C, then C is in thermal equilibrium with A. 29 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 29 The Zeroth Law of thermodynamics Zeroth Law of thermodynamics If an object A is in thermal equilibrium with B and if B is in thermal equilibrium with C, then C is in thermal equilibrium with A. 29 1.1 The states of gases