
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lectures 14 Spring,3/21/2018 Prof.,Dr.Yonghua HUANG 强 MAMLLLAMAAA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lectures 14 Spring, 3/21/2018 Prof., Dr. Yonghua HUANG http://cc.sjtu.edu.cn/G2S/site/thermo.html

△u,△h,cy,and cp relations for ideal gas ideal gas:uAT);hfT) kJ/kg.K →c,definition: du c,(T)= (ideal gas) 0 du=c,(T)dT ()-u()c(TdT (ideal gas) dh →C,definition: dT (ideal gas) The key is to ↓ find c,(T dh=c,(T)dT and cp(T) h()h()TdT Adeal gas) 上游充通大学 March 21,2018 2 SHANGHAI JLAO TONG UNIVERSITY
March 21, 2018 2 Δu, Δh, cv , and cp relations for ideal gas ideal gas: u~f(T); h~f(T) cv definition: cp definition: d ( ) (ideal gas) d v u c T T ( )d v du c T T d ( ) (ideal gas) d p h c T T d ( )d p h c T T 2 1 2 1 ( ) ( ) ( )d (ideal gas) T v T u T u T c T T 2 1 2 1 ( ) ( ) ( )d (ideal gas) T p T h T h T c T T The key is to find cv(T) and cp(T) kJ/kg·K
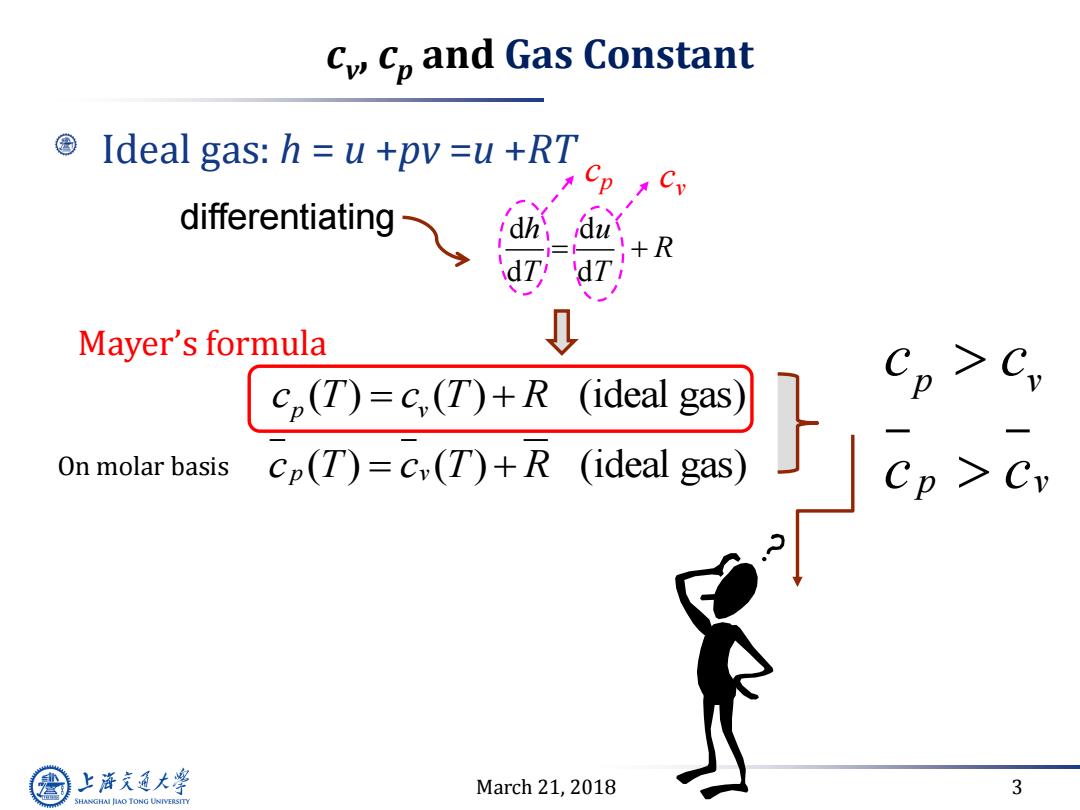
cy Cp and Gas Constant Ideal gas:h u +pv =u +RT differentiating +R dT, 'dT Mayer's formula cp(T)=c,(T)+R (ideal gas) On molar basis cp(T)=c(T)+R (ideal gas) 上游究通大学 March 21,2018 3 SHANGHAI JLAO TONG UNIVERSITY
March 21, 2018 3 cv , cp and Gas Constant Ideal gas: h = u +pv =u +RT d d d d h u R T T cp cv ( ) ( ) (ideal gas) p v c T c T R On molar basis c T c T R p v ( ) ( ) (ideal gas) p v p v c c c c Mayer’s formula differentiating
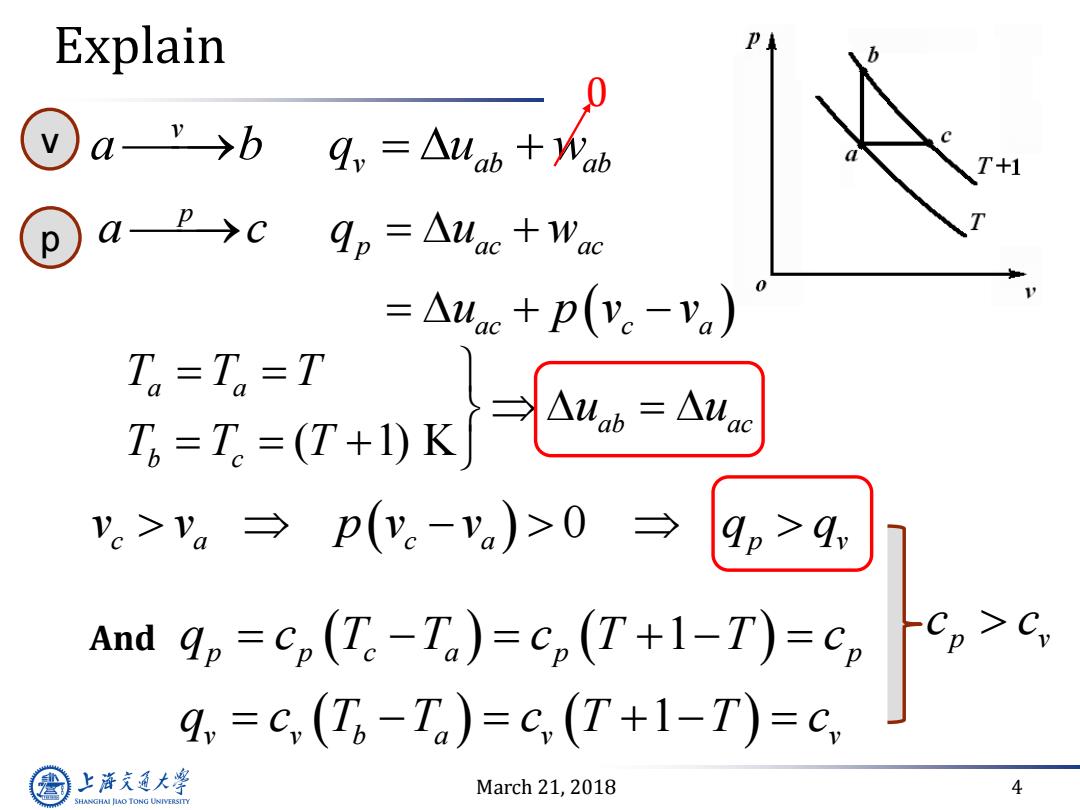
Explain ⊙a→b +1 ap→c qp=△lac+Wac =Auac+p(y。-va) 五=7-行K-= T=T=T .>。→p(.-va)>0→9,>4 And qp =cp(Te-Ta)=Cp(T+1-T)=Cp 4,=C,(T6-Ta)=C(T+1-T)=c, 上游充通大 March 21,2018 4 SHANGHAI JLAO TONG UNIVERSITY
March 21, 2018 4 v v ab ab a b q u w 0 p p ac ac ac c a a c q u w u p v v 0 c a c a p v v v p v v q q And 1 1 p p c a p p v v b a v v q c T T c T T c q c T T c T T c v p Explain ( )1 K ab a a b c a c T T u T T u T T p v c c

Question: Is c,always greater than c,at the same temperature for any phase of any substance 上游充通大学 March 21,2018 5 SHANGHAI JLAO TONG UNIVERSITY
March 21, 2018 5 Is cp always greater than cv at the same temperature ? Question: for any phase of any substance